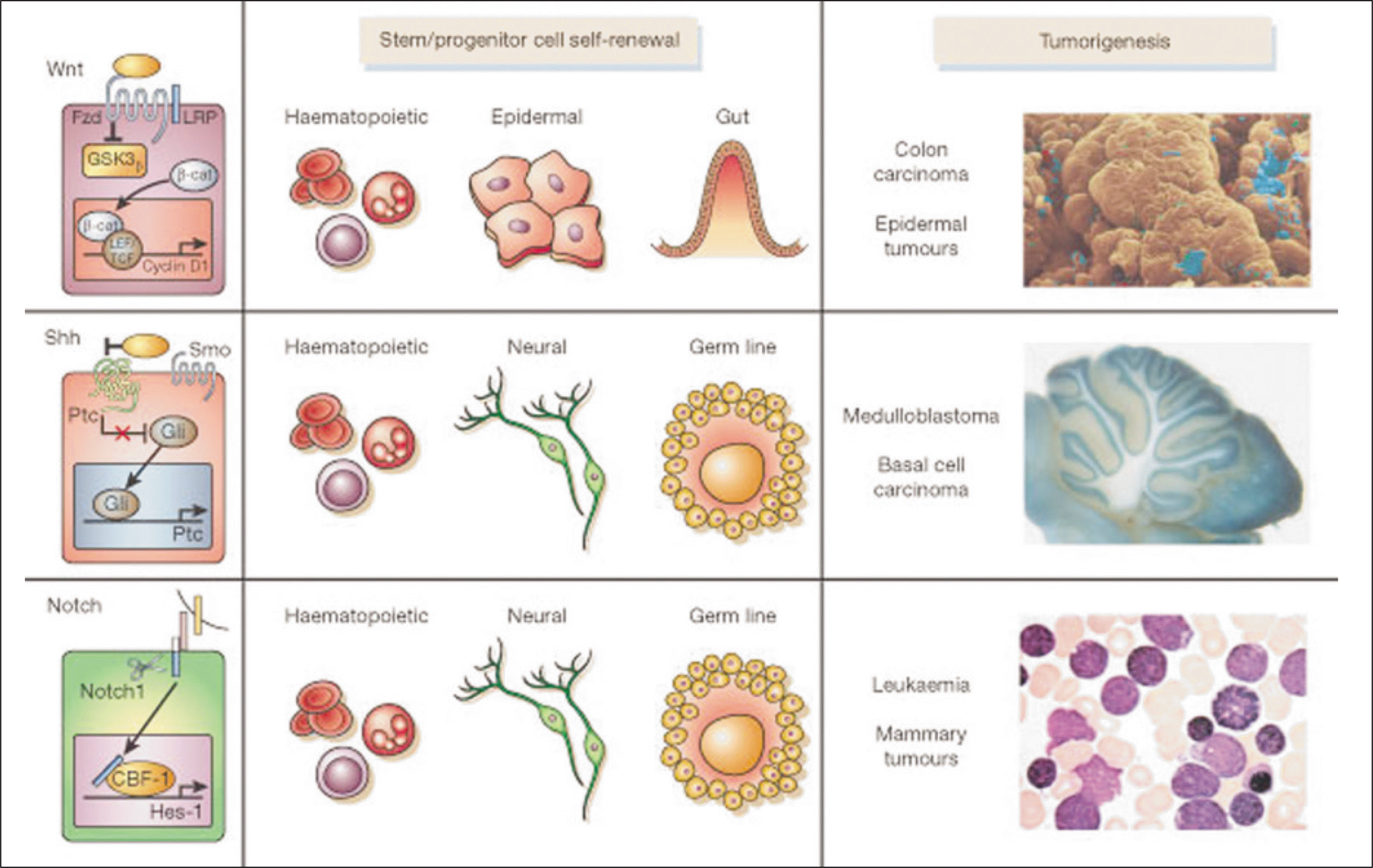
Abstract
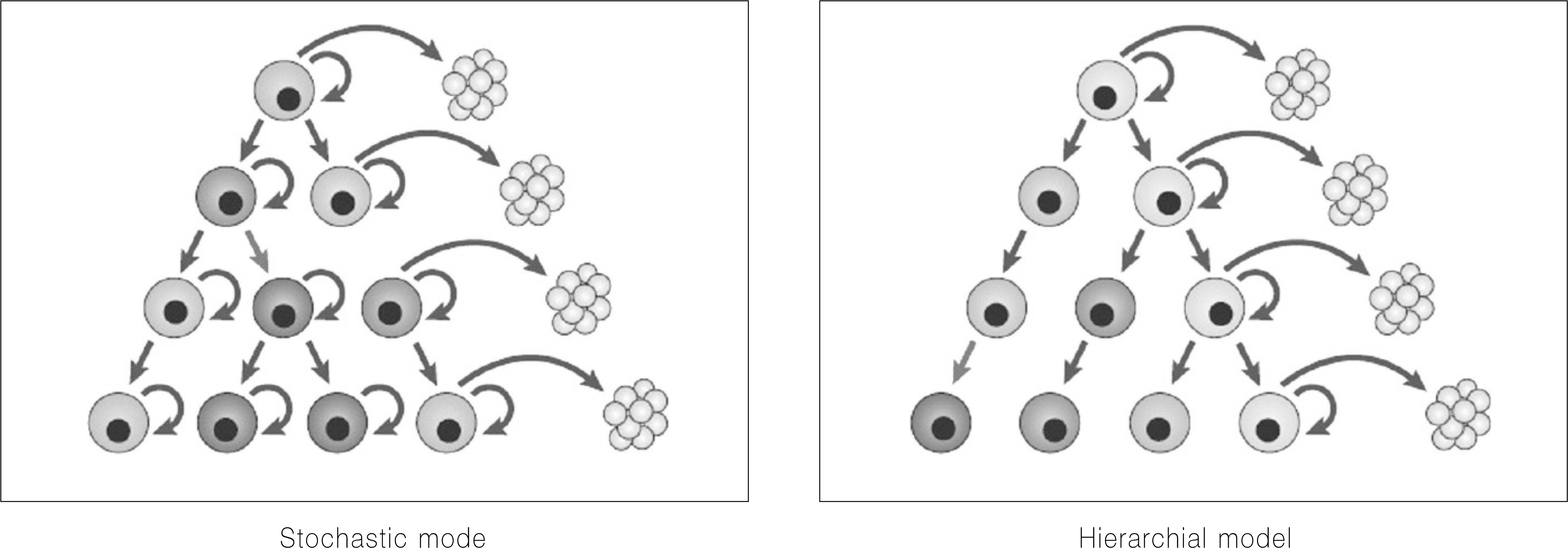
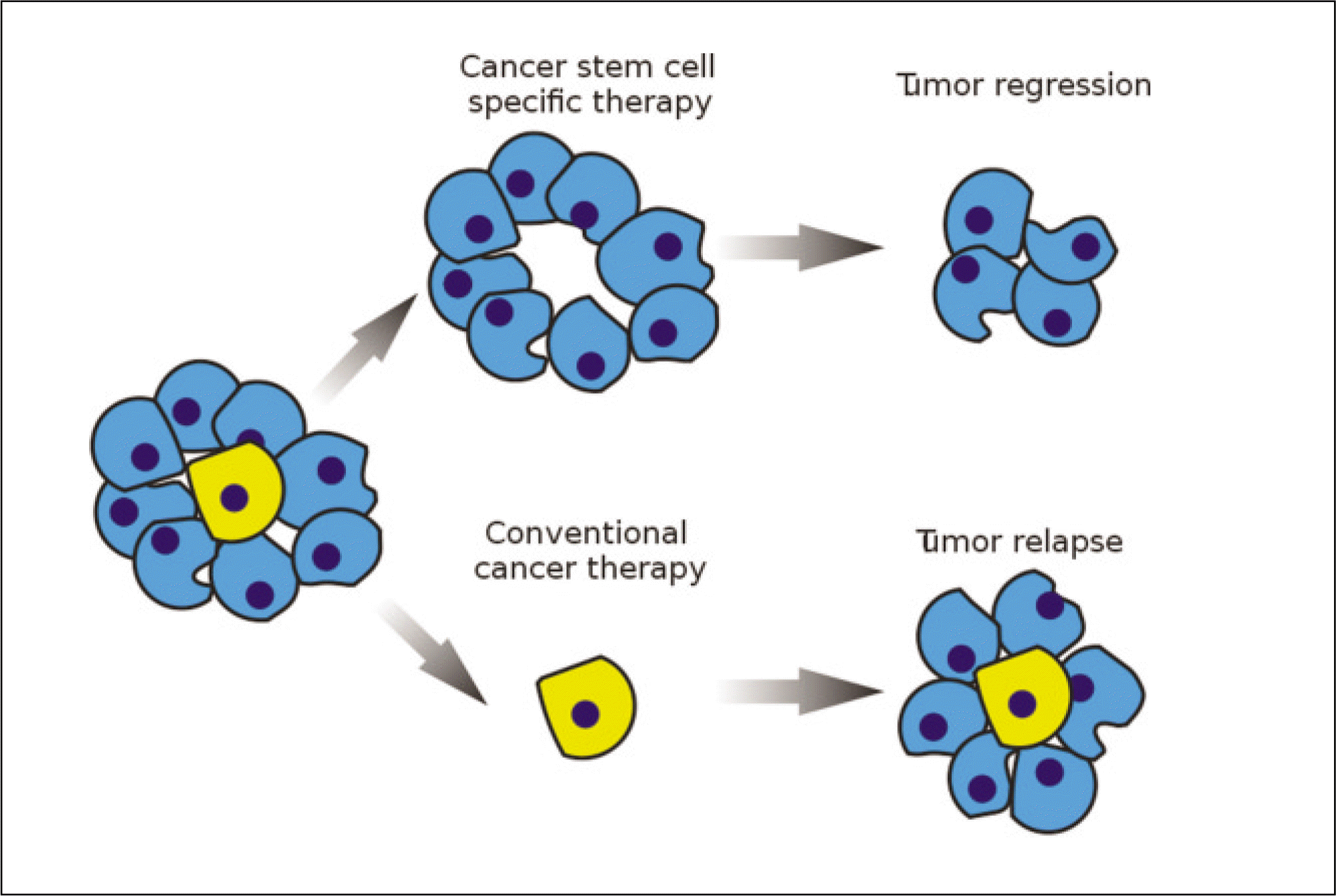
Cancer stem cells have stem cell-like features, such as the ability for self-renewal and differentiation but show unlimited growth because they have the lost normal regulation of cell growth. Cancer stem cells and normal stem cells have similar features. They show high motility, diversity of progeny, robust proliferative potential, association with blood vessels, immature expression profiles, nestin expression, epidermal growth factor (EGF)-receptor expression, phosphatase and tensin homolog (PTEN) expression, hedgehog pathway activity, telomerase activity, and Wnt pathway activity. On the other hand, with cancer cells, some of these signaling pathways are abnormally modified. In 1875, Cohnheim suggested the concept of cancer stem cells. Recently, evidence for the existence of cancer stem cells was identified. In 1994, the cancer stem cells'specific cell surface marker for leukemia was identified. Since then, other specific cell surface markers for cancer stem cells in solid tumors (e.g. breast and colon cancer) have been identified. In oral cancer, studies on cancer stem cells have been performed mainly with squamous cell carcinomas. Oral cancer specific cell surface markers, which are genes strongly expressed in oral cancer and cancer stem cell specific side populations, have been identified. Cancer stem cells are resistant to radiotherapy and chemotherapy. Therefore, to eliminate malignant tumors efficiently and reduce the recurrence rate, therapy targeting cancer stem cells needs to be performed. Currently, studies targeting the cancer stem cells'specific signaling pathways, telomerase and tumor vasculatures are being done.
Go to : 
References
1. Bray I, Brennan P, Boffetta P. Projections of alcohol- and tobacco-related cancer mortality in Central Europe Int J Cancer. 2000; 87:122–8.
2. Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004; 118:149–61.

3. Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005; 435:969–73.

5. Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000; 6:1278–81.

6. Henrique D, Hirsinger E, Adam J, Le Roux I, Pourquie O, Ish-Horowicz D, et al. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol. 1997; 7:661–70.

7. Austin J, Kimble J. Glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987; 51:589–99.

8. Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001; 2:172–80.

9. Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999; 22:103–14.

10. Zhang Y, Kalderon D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature. 2001; 410:599–604.

11. Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001; 24:385–428.

12. Gailani MR, Bale AE. Acquired and inherited basal cell carcinomas and the patched gene. Adv Dermatol. 1999; 14:261–83.
14. Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999; 21:410–3.
16. Miller SJ, Lavker RM, Sun TT. Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. Biochem Biophys Acta. 2005; 1756:25–52.

17. Crowe DL, Parsa B, Sinha UK. Relationships between stem cells and cancer stem cells. Histol Histopathol. 2004; 19:505–9.
18. Young HE, Duplaa C, Romero-Ramos M, Chesselet MF, Vourch P, Yost MJ, et al. Adult reserve stem cells and their potential for tissue engineering. Cell Biochem Biophys. 2004; 40:1–80.

19. Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004; 432:324–31.

20. Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004; 118:409–18.
21. Tsai RY. A molecular view of stem cell and cancer cell self-renewal. Int J Biochem Cell Biol 2004 36:. 684–94.
22. Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005; 353:811–22.

23. Chen CY, Chiou SH, Huang CY, Jan CI, Lin SC, Tsai ML, et al. Distinct population of highly malignant cells in a head and neck squamous cell carcinoma cell line established by xenograft model. J Biomed Sci. 2009; 16:100.

24. Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004; 51:1–28.

25. Southam CM, Brunschwig A. Quantitative studies of autotransplantation of human cancer. Cancer. 1961; 14:971–8.

26. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997; 3:730–7.

27. Reya T. Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001; 414:105–11.
29. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007; 445:111–5.

30. Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004; 103:2332–6.

31. Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996; 183:1797–806.

32. Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997; 3:1337–45.

33. Hirshmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004; 101:14228–33.
34. Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004; 101:781–6.

35. Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007; 67:4827–33.

36. Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line, Cancer Res. 2007; 67:3716–24.
37. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, et al. Ovarian cancer side population defines cells with stem cell like characteristics and mullerian inhibiting substance responsiveness, Proc Natl Acad Sci USA. 2006; 103:11154–9.
38. Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea-a paradigm shift. Cancer Res. 2006; 66:1883–90.

40. Vescovi AL, Galli R, Reynolds BA. Brain tumor stem cells. Nat Rev Cancer. 2006; 6:425–36.
41. Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet. 2005; 37:1125–9.

42. Lininger RA, Fujii H, Man YG, Gabrielson E, Tavassoli FA. Comparison of loss heterozygosity in primary and recurrent ductal carcinoma in situ of the breast. Mod Pathol. 1998; 11:1151–9.
43. Passegue′E. Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA. 2003; 100(Suppl. 1):11842–9.
44. Prindull G. Hypothesis: cell plasticity, linking embryonal stem cells to adult stem cell reservoirs and metastatic cancer cells? Exp Hematol. 2005; 33:738–46.

45. Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004; 428:564–9.

46. Janes SM, Watt FM. New roles for integrins in squamouscell carcinoma. Nat Rev Cancer. 2006; 6:175–83.

47. Boyle JO, Hakim J, Koch W, van der Riet P, Hruban RH, Roa RA, et al. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993; 53:4477–80.
48. Wallace-Brodeur RR, Lowe SW. Clinical implications of p53 mutations. Cell Mol Life Sci. 1999; 55:64–75.

49. Chen Q, Samaranayake LP, Zhen X, Luo G, Nie M, Li B. Up-regulation of Fas ligand and down regulation of Fas expression in oral carcinogenesis. Oral Oncol. 1999; 35:548–53.
50. Gastman BR, Atarshi Y, Reichert TE, Saito T, Balkir L, Rabinowich H. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999; 59:5356–64.
51. Imai Y, Sasaki T, Shinagawa Y, Akimoto K, Fujibayashi T. Expression of metastasis suppressor gene (KAI1/CD82) in oral squamous cell carcinoma and its clinicopathological significance. Oral Oncol. 2002; 38:557–61.

52. Kropveld A, Rozemuller EH, Leppers FG, Scheidel KC, de Weger RA, Koole R, et al. Sequencing analysis of RNA and DNA of exons 1 through 11 shows p53 gene alterations to be present in almost 100% of head and neck squamous cell cancers. Lab Invest. 1999; 79:347–53.
54. Mole`s JP, Watt FM. The epidermal stem cell compartment: variation in expression levels of E-cadherin and catenins within the basal layer of human epidermis. J Histochem Cytochem. 1997; 45:867–74.
55. Yokoyama K, Kamata N, Hayashi E, Hoteiya T, Ueda N, Fujimoto R, et al. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001; 37:65–71.

56. Hombach-Klonisch S, Paranjothy T, Wiechec E, Pocar P, Mustafa T, Seifert A, et al. Cancer stem cells as targets for cancer therapy: selected cancers as examples. Arch Immunol Ther Exp (Warsz). 2008; 56:165–80.

57. Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000; 10:491–500.

59. Okuyama R, Nguyen BC, Talora C, Ogawa E, Tommasi di Vignano A, Lioumi M, et al. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev Cell. 2004; 6:551–62.

60. Okuyama R, Tagami H, Aiba S. Notch signaling: Its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci. 2008; 49:187–94.

61. Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003; 33:416–21.

62. Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, Seykora J, et al. Impaired notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res. 2006; 66:7438–44.
63. The′lu J, Rossio P, Favier B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2002; 2:7.

64. Okamoto A, Chikamatsu K, Sakakura K, Hatsushika K, Takahashi G, Masuyama K. Expansion and characterization of cancer stem-like cells in squamous cell carcinoma of the head and neck. Oral Oncol. 2009; 45:633–9.

65. Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, et al. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008; 14:4085–95.

66. Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007; 104:973–8.

67. Chen JS, Pardo FS, Wang-Rodrihuez J, Chu TS, Lopez JP, Aguilera J, et al. EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope. 2006; 116:401–6.

68. Mackenzie IC. Growth of malignant oral epithelial stem cells after seeding into organotypical cultures of normal mucosa. J Oral Pathol Med. 2004; 33:71–8.

69. Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma derived cell lines. Cancer Res. 2005; 65:8944–50.
70. Costea DE, Tsinkalovsky O, Vintermyr OK, Johannessen AC, Mackenzie IC. Cancer stem cells-new and potentially important targets for the therapy of oral squamous cell carcinoma. Oral Dis. 2006; 12:443–54. Erratum in; Oral Dis 2006;12: 584.
71. Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the sidepopulation phenotype. Nat. Med. 2001; 7:1028–34.
72. Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD. A subpopulation of CD133+ cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2009; 289:151–60.
73. Jensen KB, Jones J, Watt FM. A stem cell gene expression profile of human squamous cell carcinomas. Cancer Lett. 2008; 272:23–31.

74. Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993; 73:713–24.

75. Tanemura A, Nagasawa T, Inui S, Itami S. LRIG-1 provides a novel prognostic predictor in squamous cell carcinoma of the skin: immunohistochemical analysis for 38 cases. Dermatol Surg. 2005; 31:423–30.

76. Lindstro ¨m AK, Ekman K, Stendahl U, Tot T, Henriksson R, Hedman H, et al. LRIG1 and squamous epithelial uterine cervical cancer: correlation to prognosis, other tumor markers, sex steroid hormones, and smoking. Int J Gynecol Cancer. 2008; 18:312–7.

77. Eisenmann KM, McCarthy JB, Simpson MA, Keely PJ, Guan JL, Tachibana K, et al. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat Cell Biol. 1999; 1:507–13.

78. Majumdar M, Vuori K, Stallcup WB. Engagement of the NG2 proteoglycan triggers cell spreading via rac and p130cas. Cell Signal. 2003; 15:79–84.

79. Andersen SS. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 2000; 10:261–7.

80. Ha¨yry V, Ma¨kinen LK, Atula T, Sariola H, Ma¨kitie A, Leivo I, et al. Bmi-1 expression predicts prognosis in squamous cell carcinoma of the tongue. Br J Cancer. 2010; 102:892–7.

81. Keski-Sa¨ntti H, Atula T, Hollme′n J, Ma¨kitie A, Leivo I. Predictive value of histopathologic parameters in early squamous cell carcinoma of oral tonque. Oral Oncol. 2007; 43:1007–13.
82. Spivakov M, Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Genet. 2007; 8:263–71.

83. Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. BMI-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005; 19:1432–7.

84. Kang MK, Kim RH, Kim SJ, Yip FK, Shin KH, Dimri GP, et al. Elevated BMI-1 expression is associated with dysplastic cell transformation during oral carcinogenesis and is required for cancer cell replication and survival. Br J Cancer. 2007; 96:126–33.

85. Vora HH, Shah NG, Trivedi TI, Goswami JV, Shukla SN, Shah PM. Expression of C-myc mRNA in squamous cell carcinoma of the tongue. J Surg Oncol. 2007; 95:70–8.

86. Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000; 2:84–9.

87. Zidar N, Gale N, Kojc N, Volavsek M, Cardesa A, Alos L, et al. Cadherin-catenin complex and transcription factor Snail-1 in spindle cell carcinoma of the head and neck. Virchows Arch. 2008; 453:267–74.

88. Harper LJ, Piper K, Common J, Fortune F, Mackenzie IC. Stem cell patterns in cell lines derived from head and neck squamous cell carcinoma. J Oral Pathol Med. 2007; 36:594–603.

89. Zhang P, Zhang Y, Mao L, Zhang Z, Chen W. Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Lett. 2009; 277:227–34.

90. Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2 cancer cells are similarly tumorigenic. Cancer Res. 2005; 65:6207–19.

91. Burkert J, Otto WR, Wright NA. Side populations of gastrointestinal cancers are not enriched in stem cells. J Pathol. 2008; 214:564–73.

92. Song LB, Zeng MS, Liao WT, Zhang L, Mo HY, Liu WL, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006; 66:6225–32.

93. Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005; 26:495–502.

94. Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004; 14:43–7.

95. Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005; 23:7350–60.

97. Banerji S, Los M. Important differences between topoisomerase-I and -II targeting agents. Cancer Biol Ther. 2006; 5:965–6.

98. Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol. 2006; 17:1620–4.

99. Zuse A, Prinz H, Mu¨ller K, Schmidt P, Gu¨nther EG, Schweizer F, et al. 9-Benzylidene-naphtho[2,3-b]thiophen-4-ones and benzylidene-9 (10H)-anthracenones as novel tubulin interacting agents with high apoptosis-inducing activity. Eur J Pharmacol. 2007; 575:34–45.
100. Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anticancer therapy. FASEB J. 2007; 21:3777–85.

101. Lou H, Dean M. Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene. 2007; 26:1357–60.

102. Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007; 13:4042–5.
103. Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005; 7:967–76.

105. Yang ZJ, Wechsler-Reya RJ. Hit' em where they live: targeting the cancer stem cell niche. Cancer Cell. 2007; 11:3–5.

106. Booy EP, Johar D, Maddika S, Pirzada H, Sahib MM, Gehrke I, et al. Monoclonal and bispecific antibodies as novel therapeutics. Arch Immunol Ther Exp (Warsz). 2006; 54:85–101.

107. Johnston JB, Navaratnam S, Pitz MW, Maniate JM, Wiechec E, Baust H, et al. Targeting the EGFR pathway for cancer therapy. Curr Med Chem. 2006; 13:3483–92.

108. Krzemieniecki K, Szpyt E, Rashedi I, Gawron K, Los M. Targeting of solid tumors and blood malignancies by antibody-based therapies. Cent Eur J Biol. 2006; 1:167–82.
109. Rashedi I, Panigrahi S, Ezzati P, Ghavami S, Los M. Autoimmunity and apoptosis: therapeutic implications. Curr Med Chem. 2007; 14:3139–51.
110. Peter Znamenskiy. A diagram illustrating the disctinction between cancer stem cell targeted (above) and conventional (below) cancer therapies [internet]. San Francisco, California: Wikimedia Commons;2009. Available from:. http://en.wikipedia.org/wiki/File:Cancer_stem_cells_text_resized.svg.
111. Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004; 431:707–12.

112. Farnie G, Clarke RB. Mammary stem cells and breast cancer: role of Notch signalling. Stem Cell Rev. 2007; 3:169–75.
113. Chen Z, Han ZC. STAT3: A critical transcription activator in angiogenesis. Med Res Rev. 2008; 28:185–200.

114. Houghton J. Bone-marrowderived cells and cancer: an opportunity for improved therapy. Nat Clin Pract Oncol. 2007; 4:2–3.
115. Keith WN, Thomson CM, Howcroft J, Maitland NJ, Shay JW. Seeding drug discovery: integrating telomerase cancer biology and cellular senescence to uncover new therapeutic opportunities in targeting cance stem cells. Drug Discov Today. 2007; 12:611–21.
116. Hashemi M, Ghavami S, Eshraghi M, Booy EP, Los M. Cytotoxic effects of intra and extracellular zinc chelation on human breast cancer cells. Eur J Pharmacol. 2007; 557:9–19.

117. Burek M, Maddika S, Burek CJ, Daniel PT, Schulze-Osthoff K, Los M. Apoptin-induced cell death is modulated by Bcl-2 family members and is Apaf-1 dependent. Oncogene. 2006; 25:2213–22.

118. Maddika S, Mendoza FJ, Hauff K, Zamzow CR, Paranjothy T, Los M. Cancer-selective therapy of the future: apoptin and its mechanism of action. Cancer Biol Ther. 2006; 5:10–9.

119. Ghavami S, Asoodeh A, Klonisch T, Halayko AJ, Kadkhoda K, Kroczak TJ, et al. Brevinin-2R(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J Cell Mol Med. 2008; 12:1005–22.

120. Ghavami S, Kerkhoff C, Chazin WJ, Kadkhoda K, Xiao W, Zuse A, et al. S100A8/9 induces cell death via a novel, RAGE-independent pathway that involves selective release of Smac/DIA-BLO and Omi/HtrA2. Biochim Biophys Acta. 2008; 1783:297–311.

121. Grote J, Ko¨nig S, Ackermann D, Sopalla C, Benedyk M, Los M, et al. Identification of poly(ADP-ribose) polymerase-1 and Ku70/Ku80 as transcriptional regulators of S100A9 gene expression. BMC Mol Biol. 2006; 7:48.

122. Pece S, Serresi M, Santolini E, Capra M, Hulleman E, Galimberti V, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004; 167:215–21.

123. Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002; 8:979–86.

124. Alexander HK, Booy EP, Xiao W, Ezzati P, Baust H, Los M. Selected technologies to control genes and their products for experimental and clinical purposes. Arch Immunol Ther Exp (Warsz). 2007; 55:139–49.

125. Ju KM, Jin JY, Kim HK, Nam DH. Research direction and prospect of brain cancer stem cell. Mol Cell Biol News. 2008; 20:12–8.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download