Abstract
Introduction
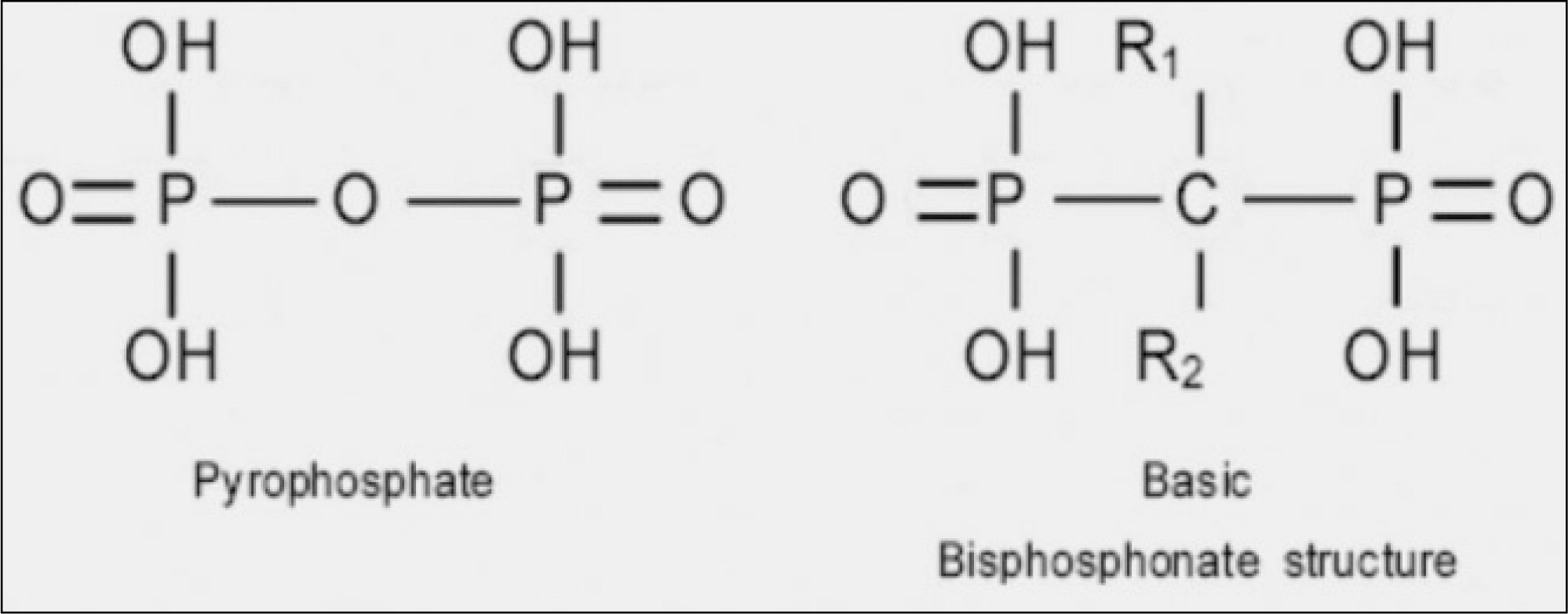
Bisphosphonates is used widely for the treatment of the Paget's disease, multiple myeloma, bone metastases of malignant tumors with the prevention of pain and their pathological fracture. However, it was recently suggested that bisphosphonates related osteonecrosis of the jaw (BRONJ) is a side effect of bisphosphonate use.
Materials and Methods
Twenty-four individuals, who were referred to the Department of Oral and Maxillofacial surgery, Dankook University Dental Hospital, were selected from those who had exposed bone associated with bisphosphonates from January, 2005 to December, 2009 according to the criteria of American Association of Oral and Maxillofacial Surgeons (AAOMS) for BRONJ. The patients group consisted of 7 males and 17 females between the age of 46 to 78 years (average 61.8 years). Each patient had panoramic imaging, computed tomography (CT), whole body bone scanning performed for a diagnosis and biopsy sampling from the necrotizing tissue. C-terminal cross-linking telopeptide of type I collagen (CTX) level of patients who had undergone surgical intervention was measured 7 days before surgery.
Results
The main cause of bone exposure was post-extraction (15), chronic periodontitis (4), persistent irritation of the denture (3). Twenty people had undergone BRONJ treatment for two to eight months except for 4 people who had to maintain the bisphosphonates treatment to prevent a metastasis and bone trabecular pain with medical treatment. When the bisphosphonate treatment was suspended at least for 3 months and followed up according to the AAOMS protocols, the exposed necrotizing bones were found to be covered by soft tissue.
Conclusion
Prevention therapy, interruption of bisphophonates for at least 3 months and cooperation with the physician for conservative treatment are the essential for treating BRONJ patient with high risk factors. The CTX level of BRONJ patients should be checked before undergoing surgical intervention. Surgical treatments should be delayed in the case of a CTX level <150 pg/mL.
References
1. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004; 62:527–34.

2. Badros A, Weikel D, Salama A, Goloubeva O, Schneider A, Rapoport A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol. 2006; 24:945–52.

3. Epstein S. Update of current therapeutic options for the treatment of postmenopausal osteoporosis. Clin Ther. 2006; 28:151–73.

4. Saad F, Lipton A. Clinical benefits and considerations of bisphosphonate treatment in metastatic bone disease. Semin Oncol. 2007; 34(6 Suppl 4):S17–23.

5. Owens G, Jackson R, Lewiecki EM. An integrated approach: bisphosphonate management for the treatment of osteoporosis. Am J Manag Care. 2007; 13(Suppl 11):S290–308. quiz S309–12.
6. Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosphonateassociated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005; 136:1658–68.
7. Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006; 144:753–61.
8. Brooks JK, Gilson AJ, Sindler AJ, Ashman SG, Schwartz KG, Nikitakis NG. Osteonecrosis of the jaws associated with use of risedronate: report of 2 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103:780–6.

9. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003; 61:1115–7.

10. Migliorati CA. Bisphosphanates and oral cavity avascular bone necrosis. J Clin Oncol. 2003; 21:4253–4.

11. Diel IJ, Bergner R, Gro¨tz KA. Adverse effects of bisphosphonates: current issues. J Support Oncol. 2007; 5:475–82.
12. Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007; 22:1479–91.
13. Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007; 65:369–76.
14. Bedogni A, Blandamura S, Lokmic Z, Palumbo C, Ragazzo M, Ferrari F, et al. Bisphosphonate-associated jawbone osteonecrosis: a correlation between imaging techniques and histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105:358–64.

16. Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonateassociated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007; 65:415–23.

18. Lehenkari PP, Kellinsalmi M, Na¨pa¨nkangas JP, Ylitalo KV, Mo¨nkko¨nen J, Rogers MJ, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002; 61:1255–62.

19. Alakangas A, Selander K, Mulari M, Halleen J, Lehenkari P, Mo¨nkko¨nen J, et al. Alendronate disturbs vesicular trafficking in osteoclasts. Calcif Tissue Int. 2002; 70:40–7.

20. Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. .;. HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356:1809–22.

21. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005; 63:1567–75.

22. Reid IR, Bolland MJ, Grey AB. Is bisphosphonateassociated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007; 41:318–20.

23. Sawatari Y, Marx RE. Bisphosphonates and bisphosphonate induced osteonecrosis. Oral Maxillofac Surg Clin North Am. 2007; 19:487–98. v-vi.

24. Lam DK, Sa ′ ndor GK, Holmes HI, Evans AW, Clokie CM. A review of bisphosphonateassociated osteonecrosis of the jaws and its management. J Can Dent Assoc. 2007; 73:417–22.
25. Yarom N, Yahalom R, Shoshani Y, Hamed W, Regev E, Elad S. Osteonecrosis of the jaw induced by orally administered bisphosphonates: incidence, clinical features, predisposing factors and treatment outcome. Osteoporos Int. 2007; 18:1363–70.

26. Phal PM, Myall RW, Assael LA, Weissman JL. Imaging findings of bisphosphonateassociated osteonecrosis of the jaws. AJNR Am J Neuroradiol. 2007; 28:1139–45.

27. Bisdas S, Chambron Pinho N, Smolarz A, Sader R, Vogl TJ, Mack MG. Biphosphonate-induced osteonecrosis of the jaws: CT and MRI spectrum of findings in 32 patients. Clin Radiol. 2008; 63:71–7.

28. Hansen T, Kunkel M, Springer E, Walter C, Weber A, Siegel E, et al. Actinomycosis of the jaws-histopathological study of 45 patients shows significant involvement in bisphosphonateassociated osteonecrosis and infected osteoradionecrosis. Virchows Arch. 2007; 451:1009–17.

29. Marx RE, Cillo JE Jr, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007; 65:2397–410.

30. Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, et al. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 2000; 66:100–3.

31. Bize R, Lamy O, Peytremann-Bridevaux I. Osteoporotic fracture in menopausal women: alendronate reduces the risk. Rev Med Suisse. 2008; 4:2703.
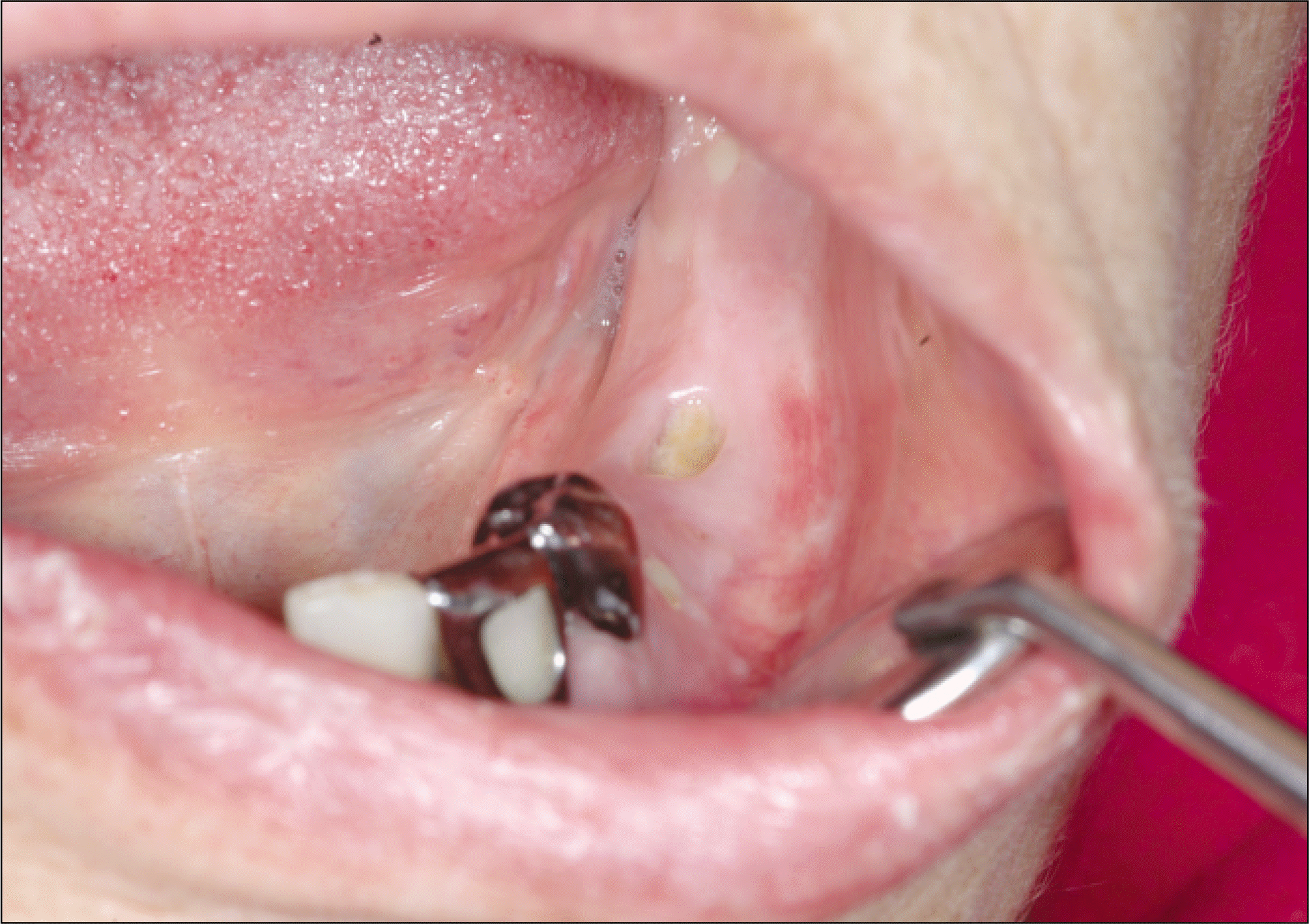
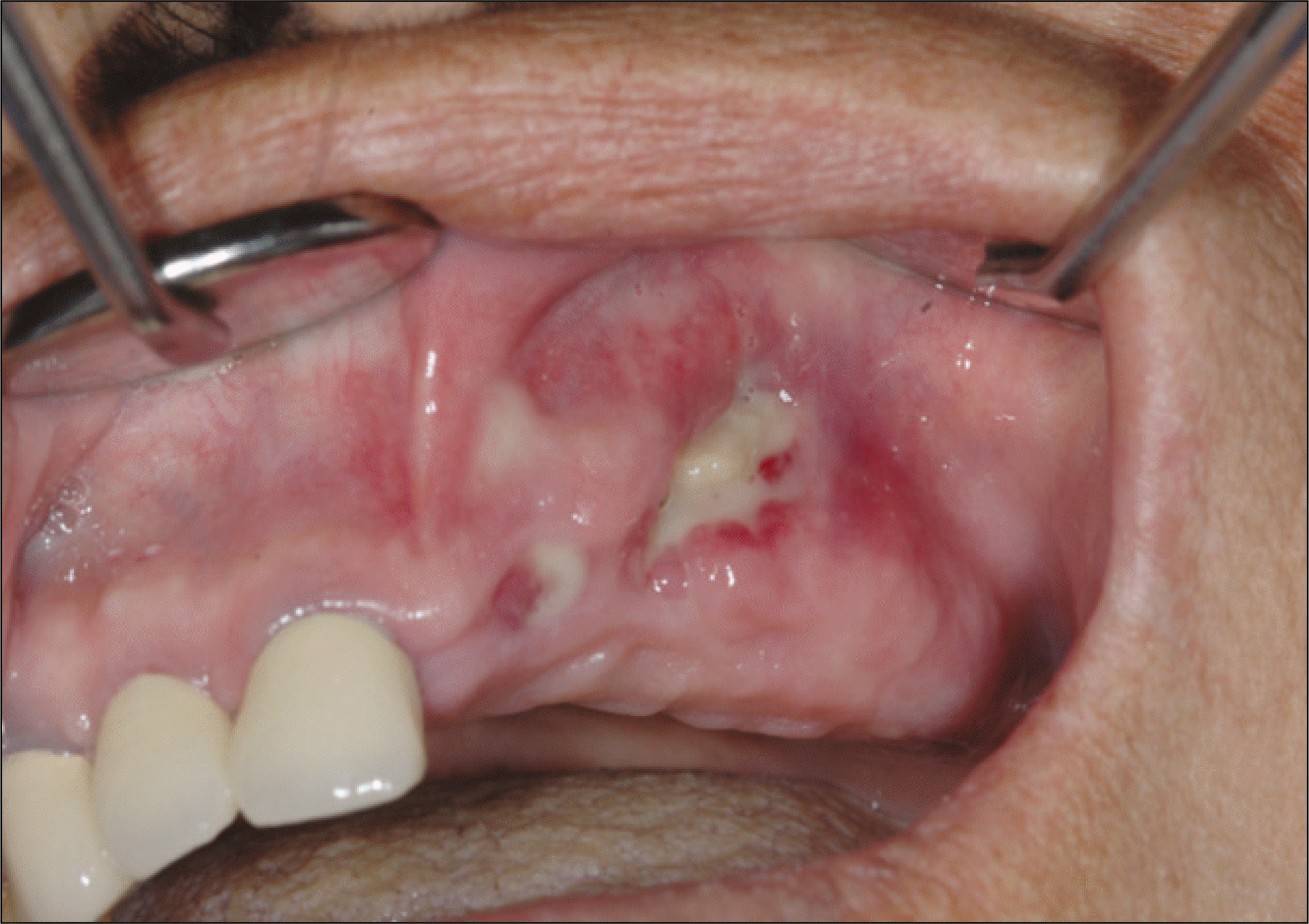
Fig. 4.
With disappearing of lamina dura and widening of periodontal space, signs of osteolysis are shown on a panoramic view.
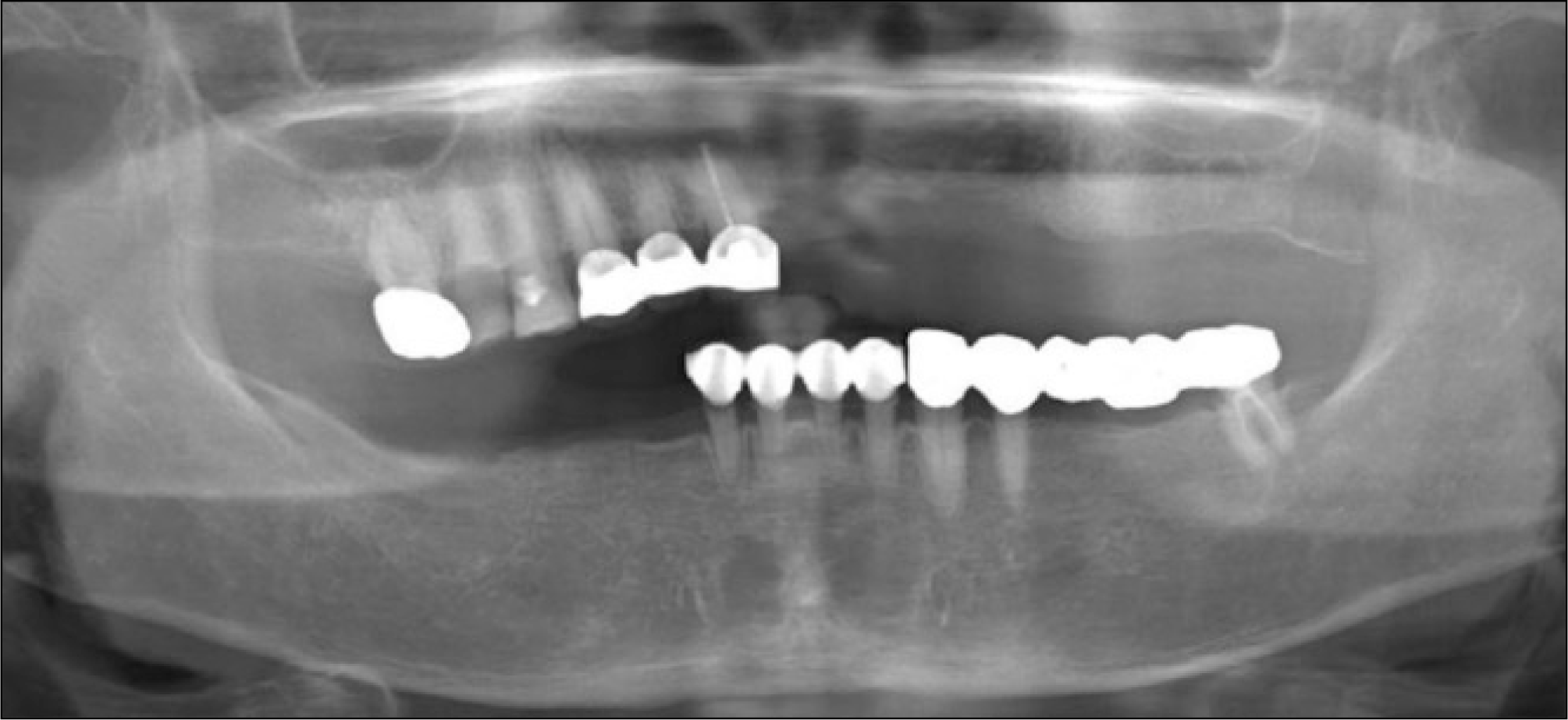
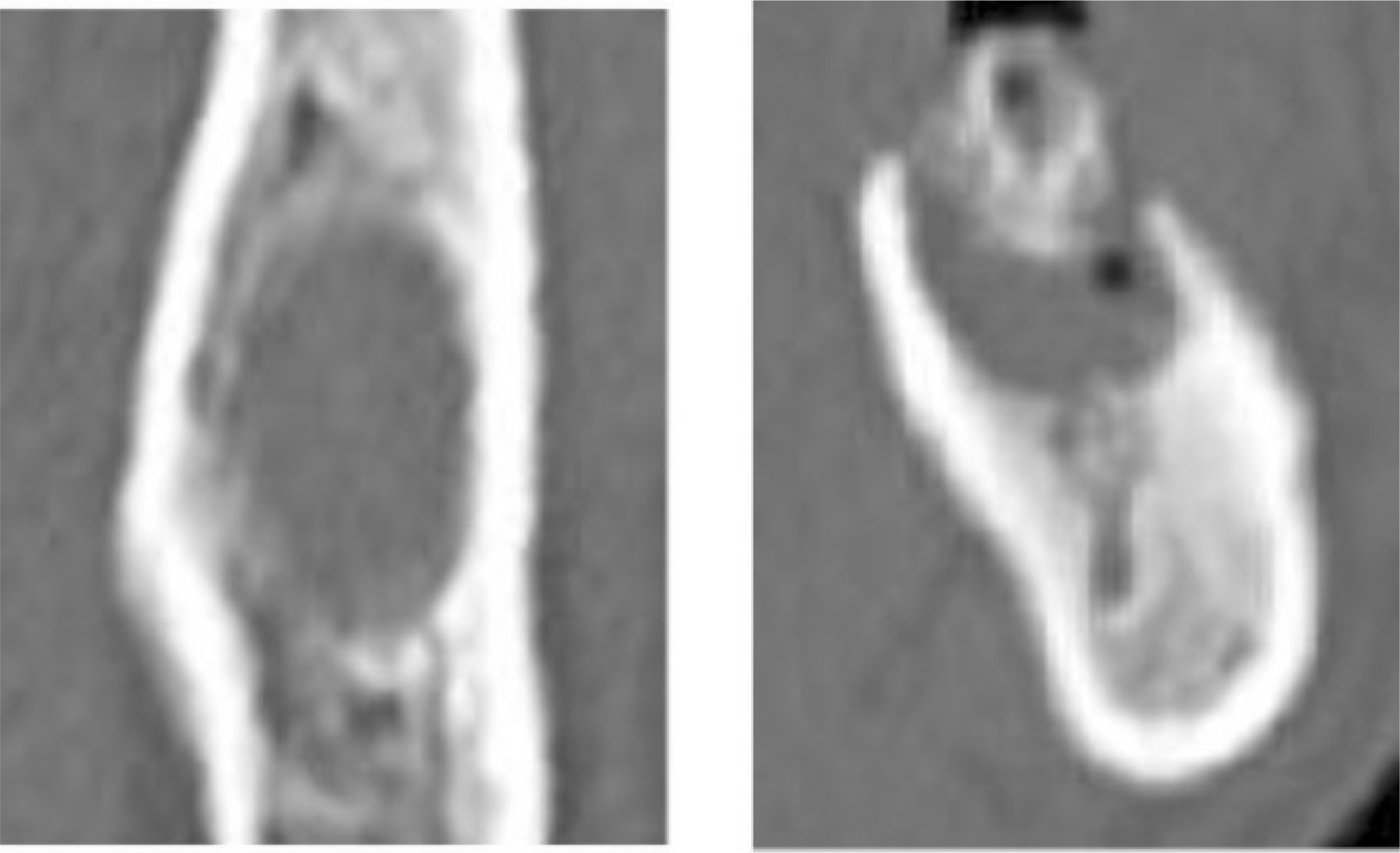
Fig. 5.
The bony trabecula deformation of the alveolar bone in the left anterior maxilla and the large sequestrum with poor cor-ticomedullary differntiation are shown on CT scan image.(CT: computed tomography)
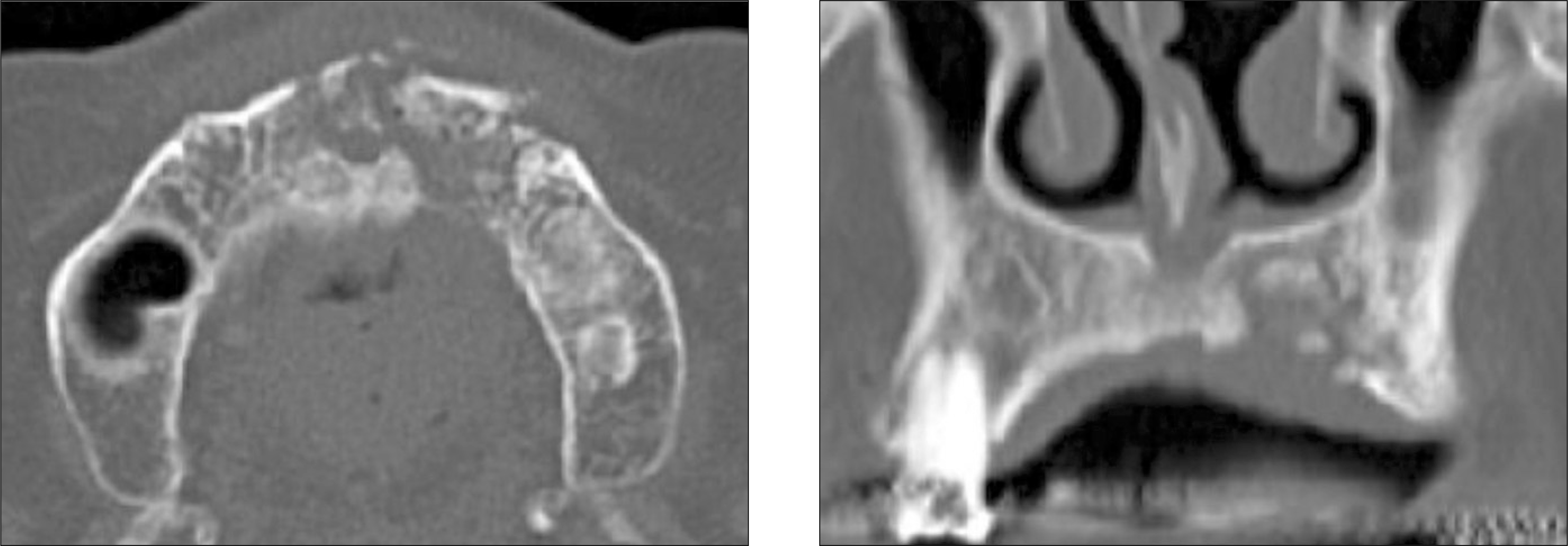
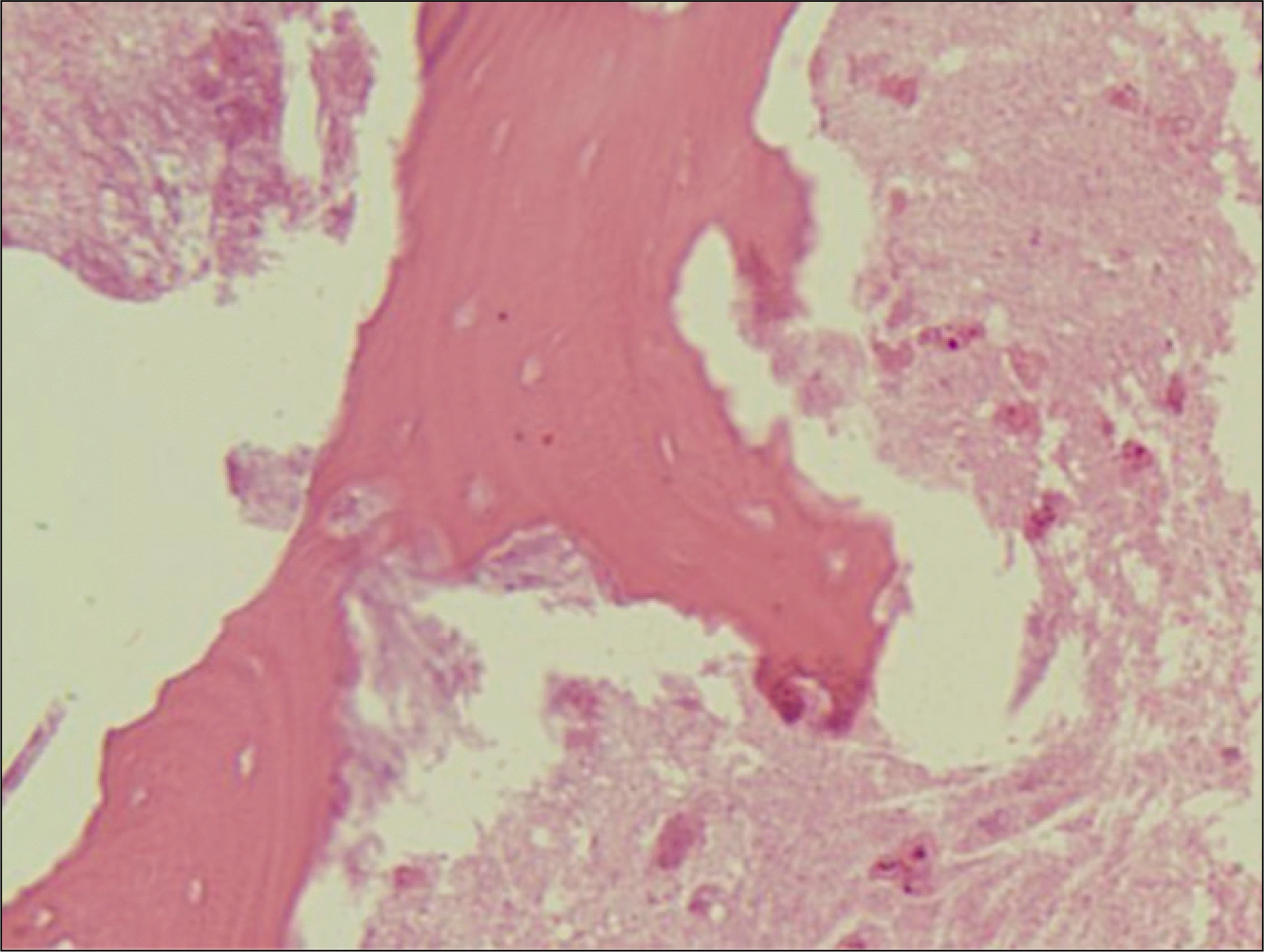
Fig. 7.
Many plasma cells, neutrophils and capillaries in granulation tissue are seen.(H&E staining, original magnification×200).
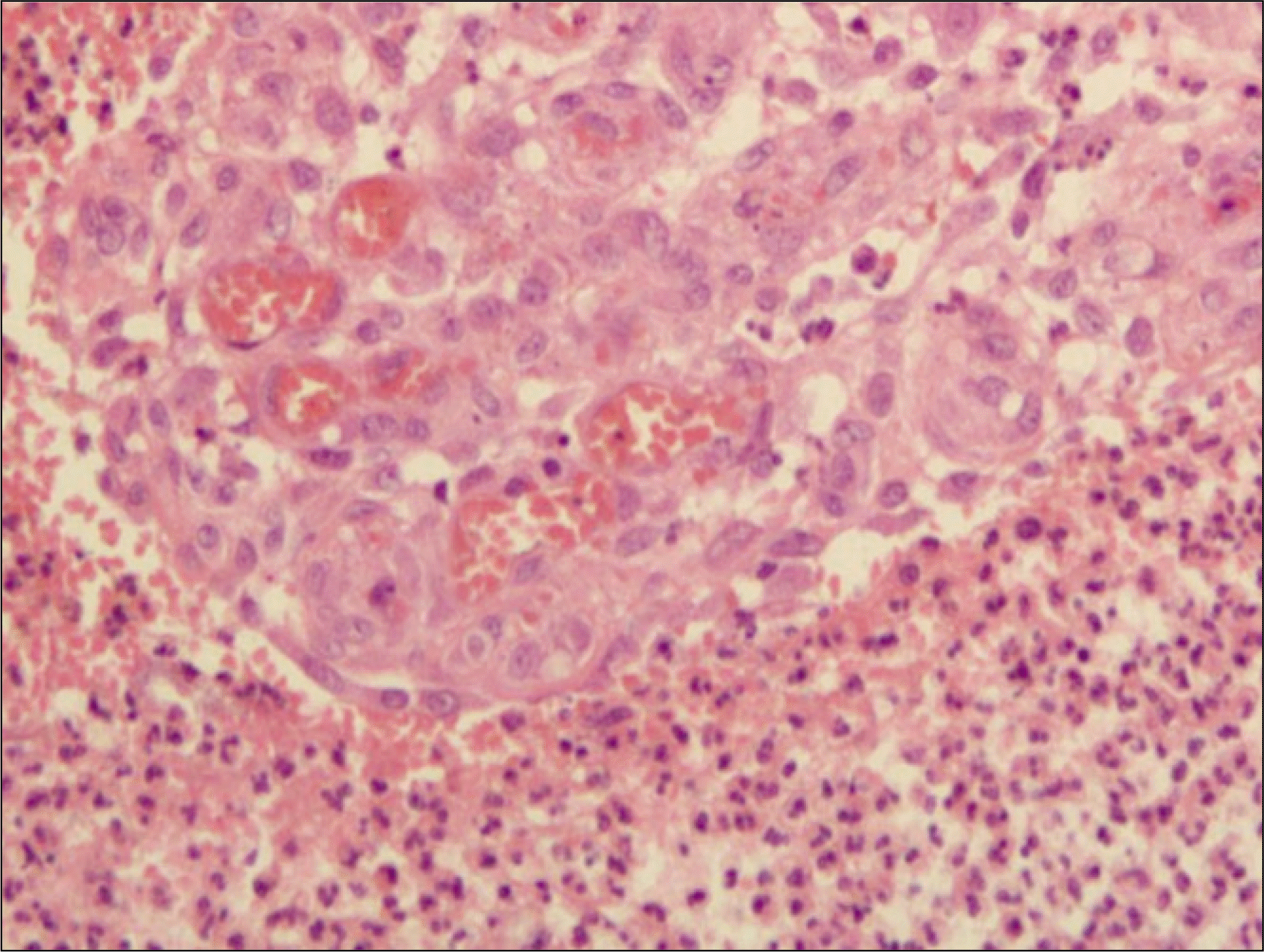
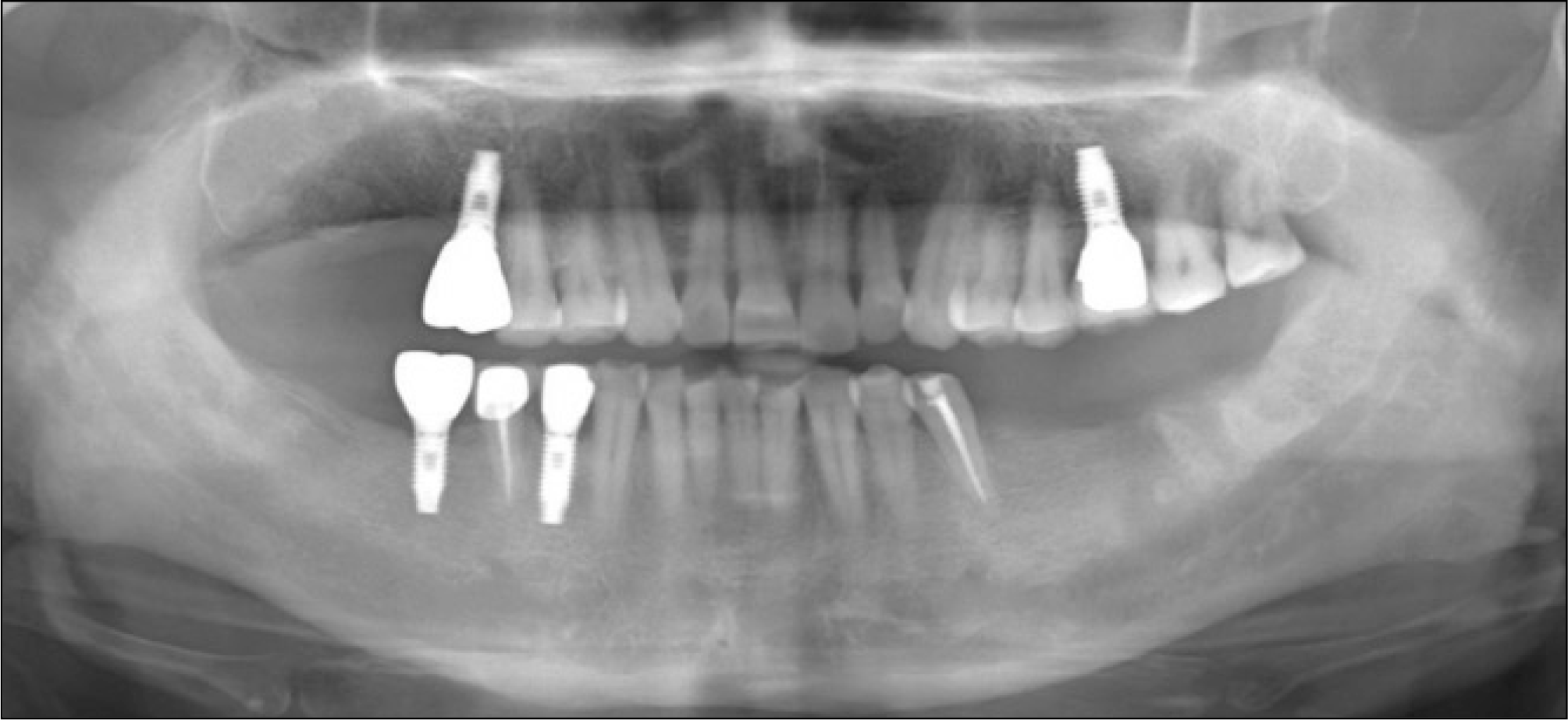
Fig. 9.
The temporal muscle flap reconstruction was performed after segmental resection of the right posterior maxilla. A complete wound healing was achieved.
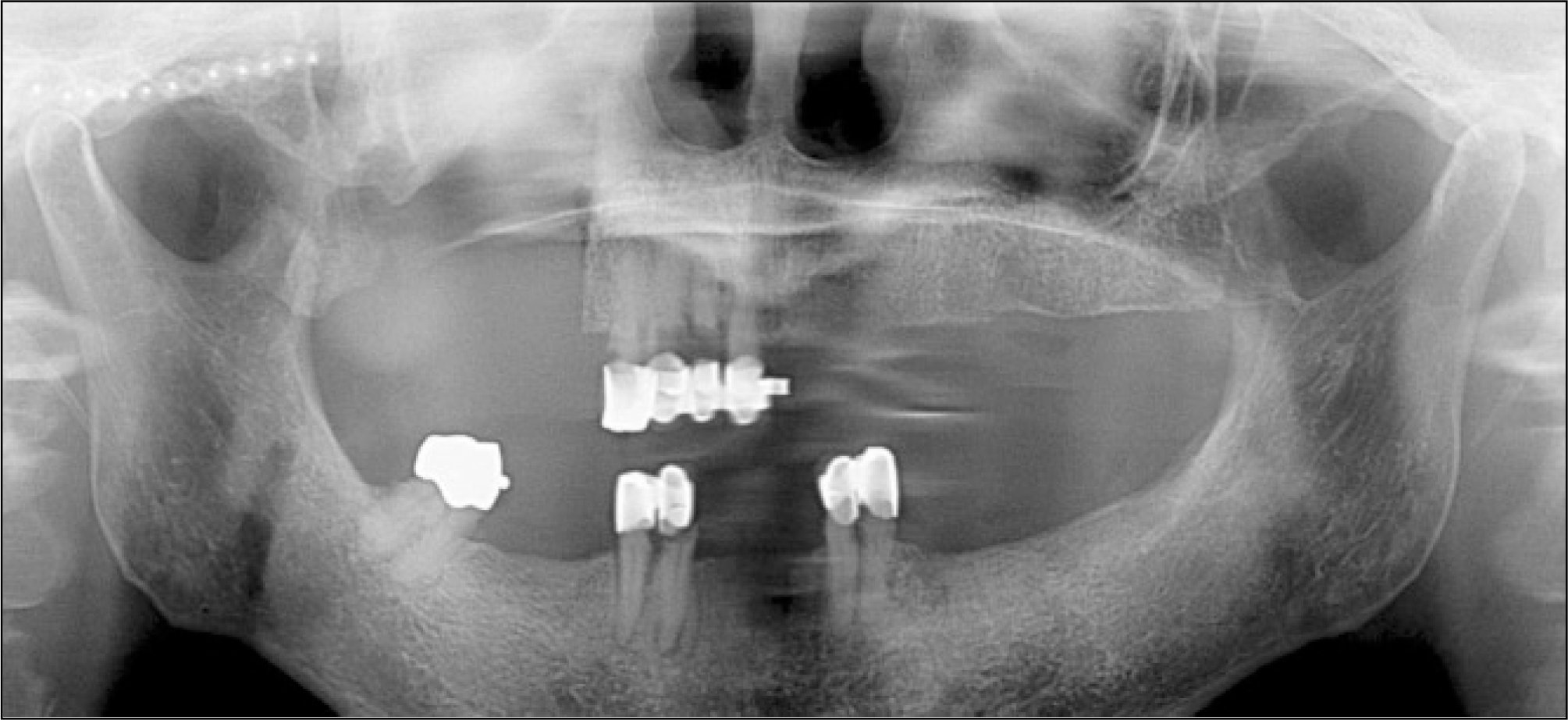
Fig. 10.
The R-plate reconstruction was performed after segmental resection of the right posterior mandible. A complete wound healing was achieved.
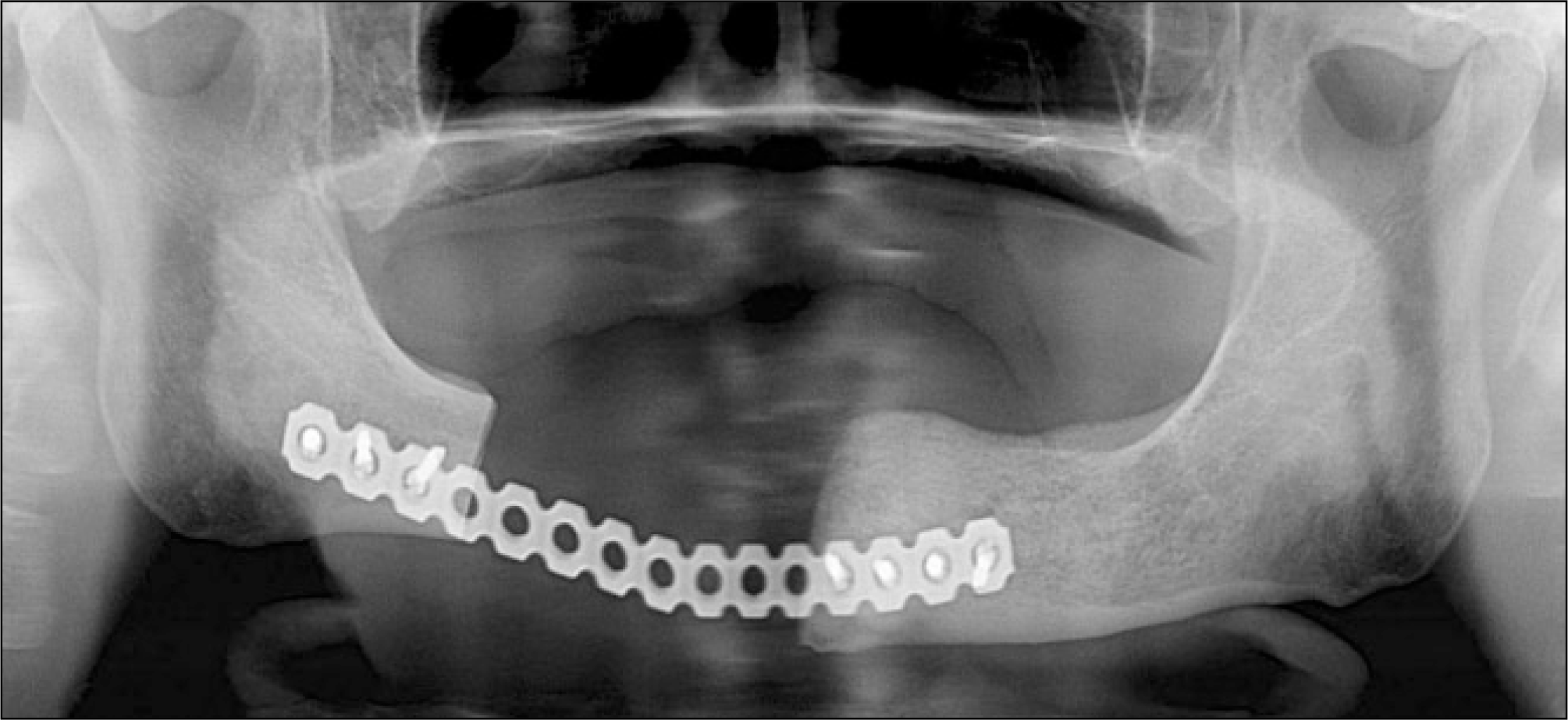
Table 1.
Patients and clinical information
Table 2.
Results of the proposed therapeutic protocol
| Patient | Stage | Cessation of BP (months) | CTX (pg/mL) | Treatment | Results |
|---|---|---|---|---|---|
| 1 | 3 | 6 | 178 | Sequestrectomy | Complete wound healing |
| 2 | 1 | 4 | 317 | No | Complete wound healing |
| 3 | 3 | 3 | 215 | Segmental resection | Complete wound healing |
| 4 | 3 | No | 168 | Sequestrectomy | Recurrence1 |
| 5 | 3 | 3 | 142 | Sequestrectomy | Progressive necrosis2 |
| 6 | 1 | 4 | 351 | No | Complete wound healing |
| 7 | 3 | 3 | 135 | Sequestrectomy | Complete wound healing |
| 8 | 3 | 3 | 113 | Sequestrectomy | Recurrence |
| 9 | 2 | 8 | 215 | Debridement | Complete wound healing |
| 10 | 1 | 3 | 273 | No | Complete wound healing |
| 11 | 3 | No | 162 | Sequestrectomy | Recurrence |
| 12 | 2 | 5 | 261 | Debridement | Complete wound healing |
| 13 | 2 | No | 183 | Debridement | Recurrence |
| 14 | 3 | 3 | 84 | No | Progressive necrosis |
| 15 | 3 | 6 | 278 | Sequestrectomy | Complete wound healing |
| 16 | 2 | 2 | 314 | Debridement | Progressive necrosis |
| 17 | 1 | 4 | 372 | No | Complete wound healing |
| 18 | 3 | 8 | 236 | Sequestrectomy | Complete wound healing |
| 19 | 3 | 3 | 121 | Sequestrectomy | Recurrence |
| 20 | 3 | 4 | 328 | Sequestrectomy | Complete wound healing |
| 21 | 2 | No | 271 | Debridement | Progressive necrosis |
| 22 | 1 | 5 | 325 | No | Complete wound healing |
| 23 | 2 | 3 | 96 | No | Progressive necrosis |
| 24 | 2 | 6 | 217 | Debridement | Complete wound healing |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download