Abstract
Introduction
Development of carcinoma on oral tongue may cause bilateral cervical lymph node metastasis, rapid invasion and growth of the cancer cells due to rich blood supply in muscle tissues. It is not only difficult to develop an animal experimental model, but also to proceed follow-up research after the development of such model as the induction of cancer lead to difficulty in taking nutrition for the experimental animals that often causes early death.
Materials and Methods
IIn this study, author have transplanted YD-10Bmod cells into nude mouse oral tongues with different cells number (5×104, 5×105, 5×106 cells/mouse) and observed the development aspect of oral tongue cancers.
Results
The cancer developed from orthotopic transplantation of YD-10Bmod cells into nude mouse oral tongue show invasion and central necrosis of the tumor, similar to the cancers developed human oral tongue cancer. The difference in tumor size and the time of central necrosis development depending on the number of transplanted tumor cells shows the feasibility of extending the survival period of the nude mouse by limiting the transplanted tumor cells to <5×104 cells/mouse or under per nude mouse.
Go to : 
References
1. MJ Kim. Oral Cancer. 1st ed.Seoul: Jisung Publishing;2002.
2. Zelefsky MJ, Harrison LB, Fass DE, Armstrong J, Spiro RH, Shah JP, et al. Postoperative radiotherapy for oral cavity cancers: impact of anatomic subsite on treatment outcome. Head Neck. 1990; 12:470–5.

3. Salley JJ. Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J Dent Res. 1954; 33:253–62.

4. Morris AL. Factors influencing experimental carcinogensis in the hamster cheek pouch. J Dent Res. 1961; 40:3–15.
5. Hendrickson EA. The SCID mouse: relevance as an animal model system for studying human disease. Am J Pathol. 1993; 143:1511–22.
6. Kubota T. Metastatic models of human cancer xenografted in the nude mouse: the importance of orthotopic transplantation. J Cell Biochem. 1994; 56:4–8.

7. Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998–1999; 17:279–84.
8. Lee EJ, Kim J, Lee SA, Kim EJ, Chun YC, Ryu MH, et al. Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoepidermoid carcinoma cells. Exp Mol Med. 2005; 37:379–90.

9. Kim JH, Hwang YS, Kim HS, Nam W, Cha IH. An atopic nude mouse model of oral cancer cell line. J Korean Assoc Oral Maxillofac Surg. 2009; 35:74–82.
10. Johnson N. Global Epidemiology. Shah JP, Johnson NW, Batsakis JG, editors. Oral Cancer. London: Martin Dunitz Publication;2003. p. 7–22.
11. Henson B, Li F, Coatney DD, Carey TE, Mitra RS, Kirkwood KL, et al. An orthotopic floor-of-mouth model for locoregional growth and spread of human squamous cell carcinoma. J Oral Pathol Med. 2007; 36:363–70.

12. Jang MJ, Lee JH, Kim CH. An experimental study on the therapeutic effect of ABNOBAviscum. on oral squamous cell carcinoma xenografted in nude mice. J Korean Assoc Maxillofac Plast Reconstr Surg. 2003; 25:383–97.
13. Park HK, Kim YK. Making in vivo model to study about human oral cancer (I). J Korean Assoc Maxillofac Plast Reconstr Surg. 1997; 19:300–10.
14. Qiu C, Wu H, He H, Qiu W. A cervical lymph node metastatic model of human tongue carcinoma: Serial and orthotopic transplantation of histologically intact patient specimens in nude mice. J Oral Maxillofac Surg. 2003; 61:696–700.

15. Lou E, Kellman RM, Hutchison R, Shillitoe EJ. Clinical and pathological features of the murine AT-84 orthotopic model of oral cancer. Oral Dis. 2003; 9:305–12.

16. Shklar G. Oral cancer: the diagnosis, therapy, management, and rehabilitation of the oral cancer patient. Philadelphia: W.B. Saunders;1984. p. 138–43.
Go to : 
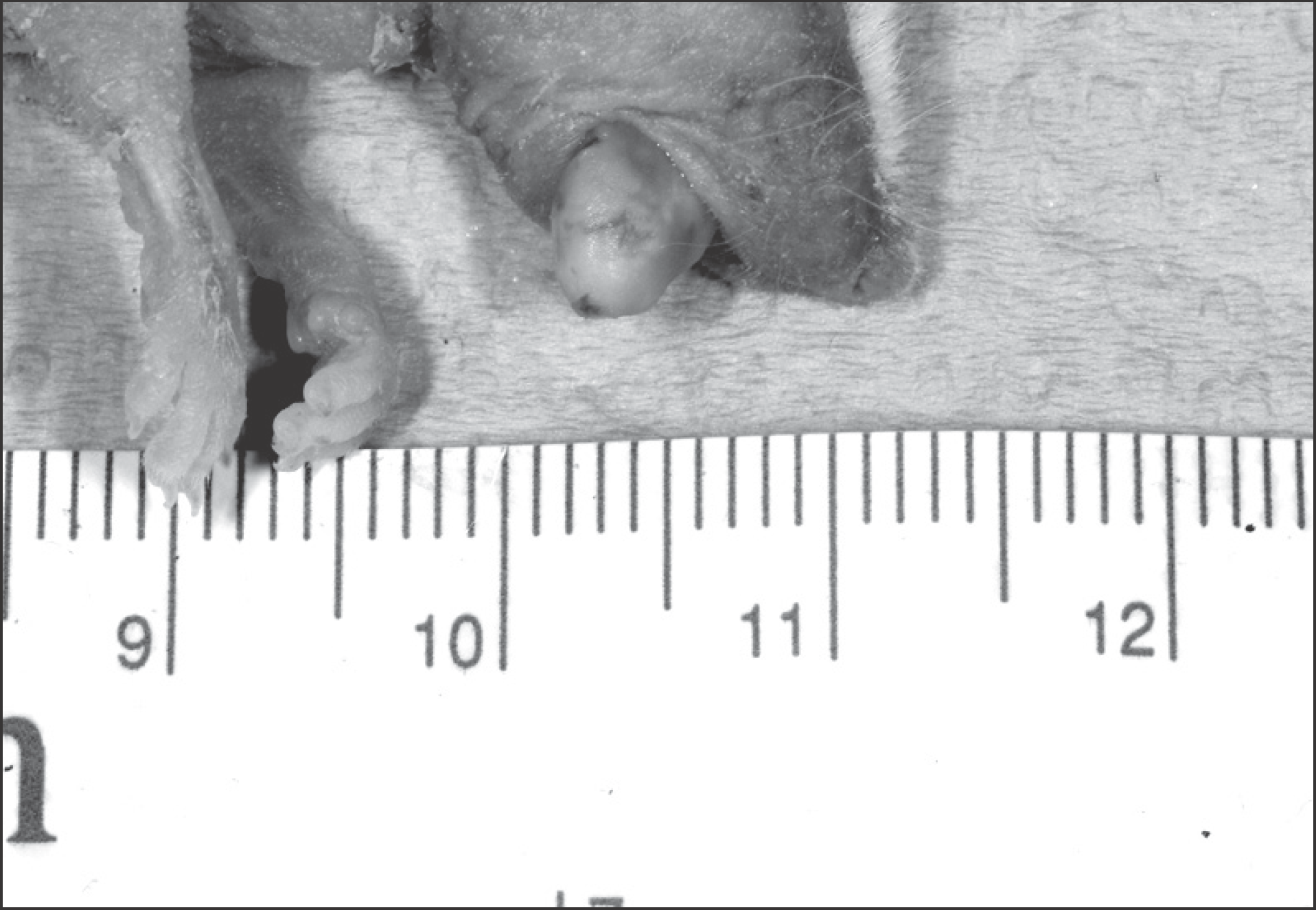 | Fig. 1.Tumor formation on lateral border of tongue. Jae-Seung Chung et al: An orthotopic nude mouse model of tongue carcinoma. J Korean Assoc Oral Maxillofac Surg 2011
|
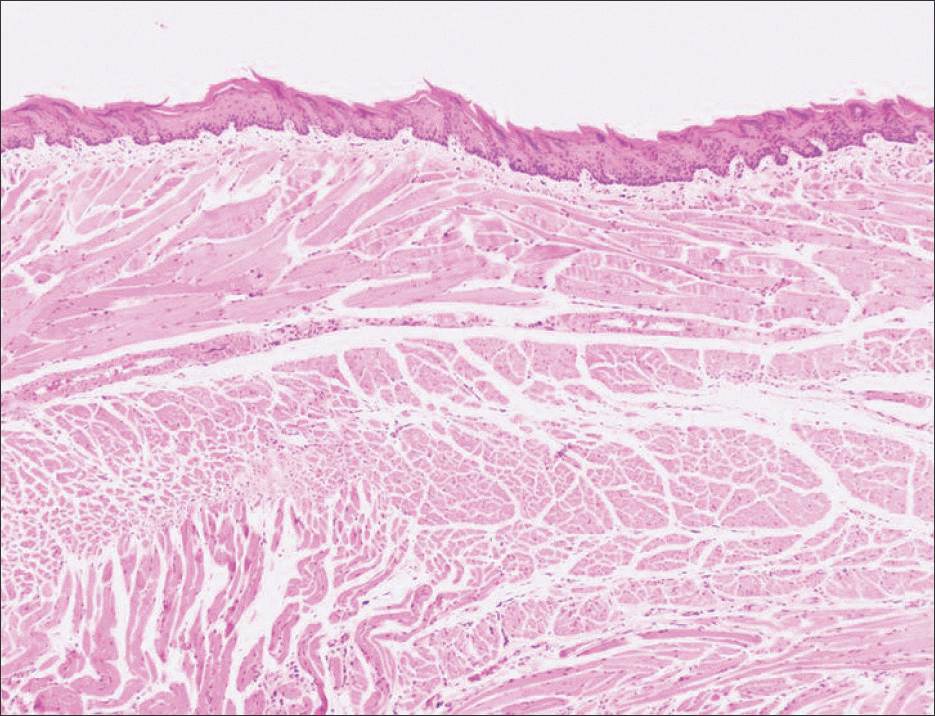 | Fig. 2.There is no tumor formation by subcutaneous inoculation of media only in control group (3 weeks, H&E staining, ×40). Jae-Seung Chung et al: An orthotopic nude mouse model of tongue carcinoma. J Korean Assoc Oral Maxillofac Surg 2011
|
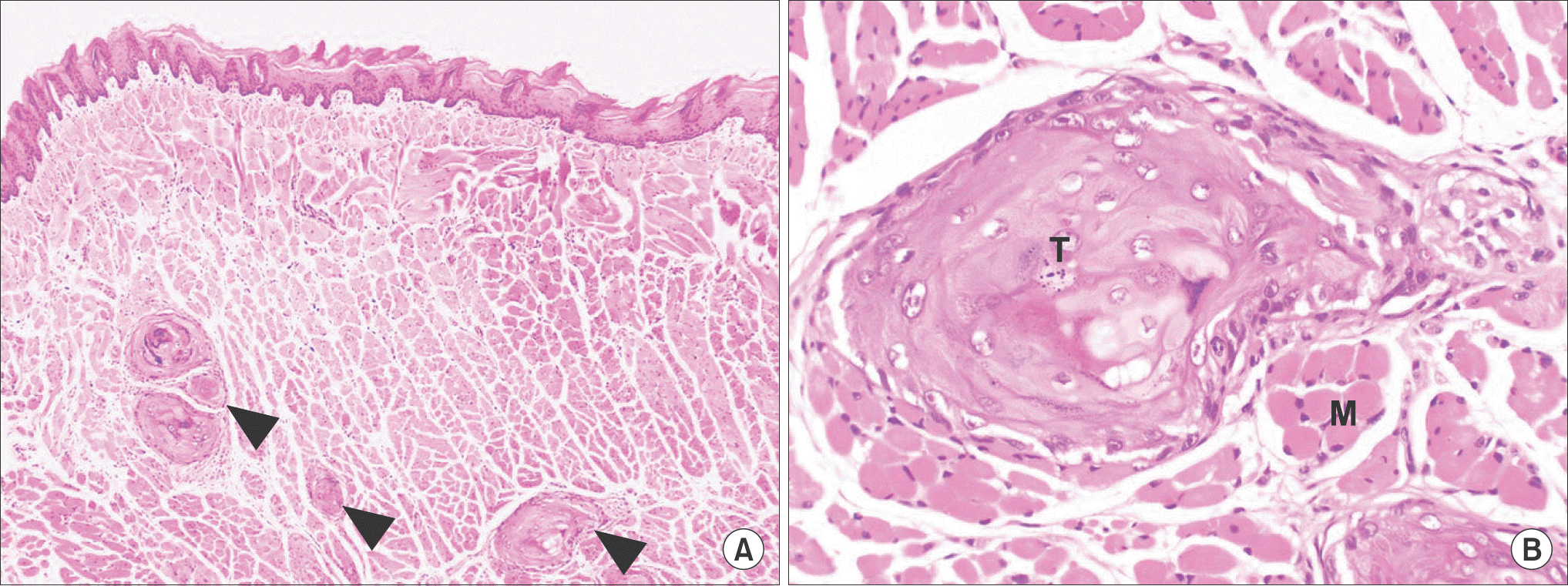 | Fig. 3.The histopathologic findings of tumor (arrowheads) of YD-10Bmod cells (5×104 cells/mouse, 3 weeks). The tumor (T) showed invasive growth into surrounding muscle tissue (M) (H&E staining, A: ×40, B: ×200). Jae-Seung Chung et al: An orthotopic nude mouse model of tongue carcinoma. J Korean Assoc Oral Maxillofac Surg 2011
|
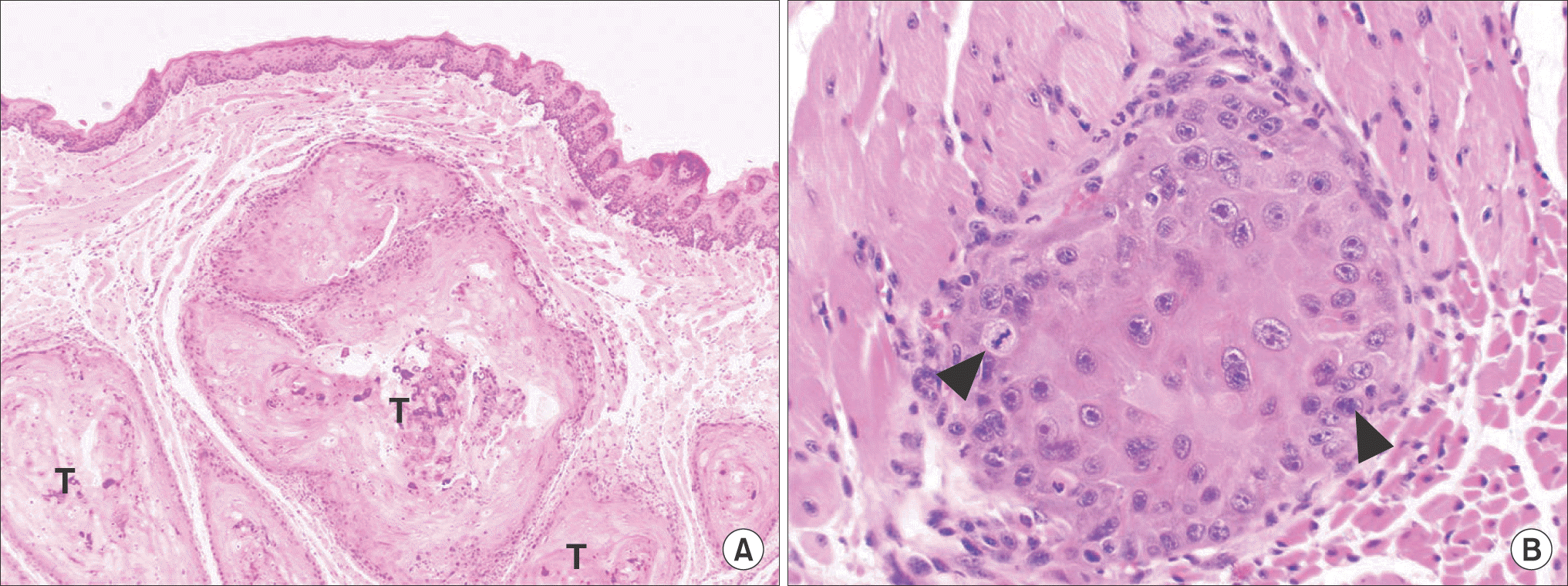 | Fig. 4.The histopathologic findings of tumor of YD-10Bmod cells (5×105 cells/mouse, 3 weeks). The tumor (T) showed invasive growth into surrounding tissue. The tumor cells showed cellular dysplasia and abnormal mitosis (arrowheads) (H&E staining, A: ×40, B: ×200). Jae-Seung Chung et al: An orthotopic nude mouse model of tongue carcinoma. J Korean Assoc Oral Maxillofac Surg 2011
|
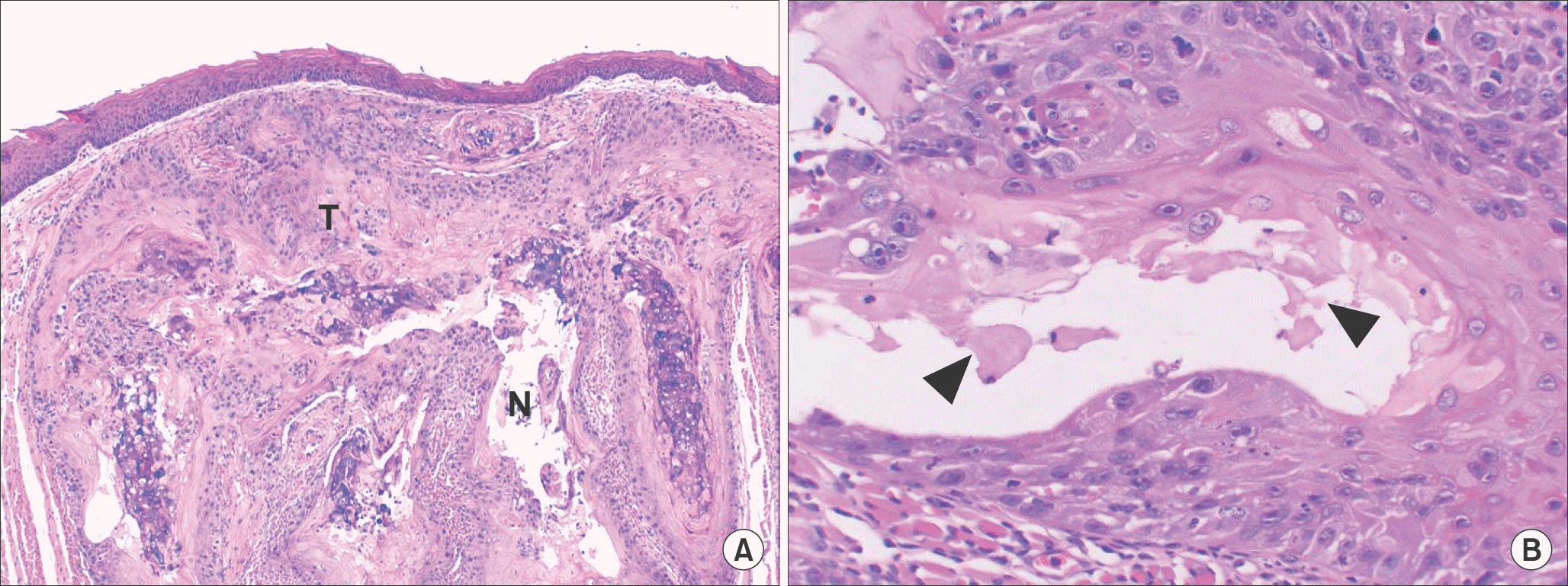 | Fig. 5.The histopathologic findings of tumor of YD-10Bmod cells (5×106 cells/mouse, 3 weeks). The tumor (T) showed invasive growth into surrounding tissue. The tumor showed cellular pleomorphism and central necrosis (N, arrowheads) (H&E staining, A: ×40, B: ×200). Jae-Seung Chung et al: An orthotopic nude mouse model of tongue carcinoma. J Korean Assoc Oral Maxillofac Surg 2011
|
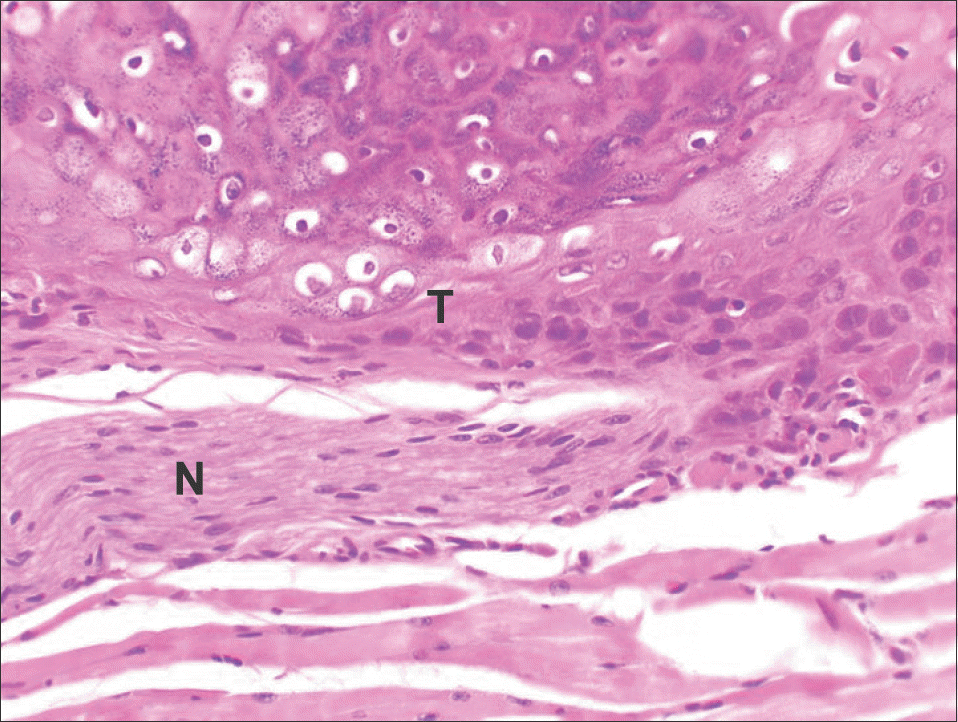 | Fig. 6.The histopathologic findings of tumor of YD-10Bmod cells (5×106 cells/mouse, 2 weeks). The tumor (T) showed invasive growth into peripheral nerve sheath (N) (H&E staining, ×200). Jae-Seung Chung et al: An orthotopic nude mouse model of tongue carcinoma. J Korean Assoc Oral Maxillofac Surg 2011
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download