Abstract
Introduction
Dental implants are used routinely with high success rates in generally healthy individuals. By contrast, their use in patients with diabetes mellitus is controversial because altered bone healing around implants has been reported. This study examined the bone healing response around titanium implants placed immediately in rats with controlled and uncontrolled diabetes.
Materials and Methods
Twenty rats were divided into the control, insulin-treated and diabetic groups. The rats received streptozotocin (60 mg/kg) to induce diabetes; animals in the insulin-treated group also received three units of subcutaneous slow-release insulin. A titanium implant (1.2×3 mm) was placed in the extraction socket of the maxillary first molar and bone block was harvested at 1, 2 and 4 weeks.
References
1. Balshi TJ, Wolfinger GJ. Dental implants in the diabetic patient: a retrospective study. Implant Dent. 1999; 8:355–9.
2. Thorstensson H, Kuylenstierna J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulindependent diabetics. J Clin Periodontol. 1996; 23:194–202.

3. Lazzara RJ. Immediate implant placement into extraction sites: surgical and restorative advantages. Int J Periodontics Restorative Dent. 1989; 9:332–43.
4. Wagenberg BD, Ginsburg TR. Immediate implant placement on removal of the natural tooth: retrospective analysis of 1,081 implants. Compend Contin Educ Dent. 2001; 22:399–404. 406, 408 passim; quiz 412.
5. Becker W, Goldstein M. Immediate implant placement: treatment planning and surgical steps for successful outcome. Periodontol 2000. 2008; 47:79–89.

6. Marder MZ. Medical conditions affecting the success of dental implants. Compend Contin Educ Dent. 2004; 25:739–42. 744, 746 passim; quiz 772, 795.
7. Oczakir C, Balmer S, Mericske-Stern R. Implant-prosthodontic treatment for special care patients: a case series study. Int J Prosthodont. 2005; 18:383–9.
8. McMahon MM, Bistrian BR. Host defenses and susceptibility to infection in patients with diabetes mellitus. Infect Dis Clin North Am. 1995; 9:1–9.

9. Kotsovilis S, Karoussis IK, Fourmousis I. A comprehensive and critical review of dental implant placement in diabetic animals and patients. Clin Oral Implants Res. 2006; 17:587–99.

10. Mombelli A, Cionca N. Systemic diseases affecting osseointegration therapy. Clin Oral Implants Res. 2006; 17(Suppl 2):97–103. Erratum in: Clin Oral Implants Res 2006;17: 746.

11. Olson JW, Shernoff AF, Tarlow JL, Colwell JA, Scheetz JP, Bingham SF. Dental endosseous implant assessments in a type 2 diabetic population: a prospective study. Int J Oral Maxillofac Implants. 2000; 15:811–8.
12. van Steenberghe D, Jacobs R, Desnyder M, Maffei G, Quirynen M. The relative impact of local and endogenous patient-related factors on implant failure up to the abutment stage. Clin Oral Implants Res. 2002; 13:617–22.

13. Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent. 2000; 20:366–73.
14. Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000; 5:157–65.

15. Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants. 1998; 13:620–9.

16. Fiorellini JP, Nevins ML, Norkin A, Weber HP, Karimbux NY. The effect of insulin therapy on osseointegration in a diabetic rat model. Clin Oral Implants Res. 1999; 10:362–8.

17. McCracken MS, Aponte-Wesson R, Chavali R, Lemons JE. Bone associated with implants in diabetic and insulin-treated rats. Clin Oral Implants Res. 2006; 17:495–500.

18. Takeshita F, Iyama S, Ayukawa Y, Kido MA, Murai K, Suetsugu T. The effects of diabetes on the interface between hydroxyapatite implants and bone in rat tibia. J Periodontol. 1997; 68:180–5.

19. Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiya P. Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 2003; 12:242–51.

21. Taylor AM, Sharma AK, Avasthy N, Duguid IG, Blanchard DS, Thomas PK, et al. Inhibition of somatomedin-like activity by serum from streptozotocin-diabetic rats: prevention by insulin treatment and correlation with skeletal growth. Endocrinology. 1987; 121:1360–5.

22. Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest. 1980; 66:709–19.

23. Jeong SY, Shin SH, Kim UK, Park BS, Chung IK. The study of bone mineral density in the mandible of streptozotocin-induced diabetic rats. J Korean Assoc Oral Maxillofac Surg. 2002; 28:95–102.
24. Goodman WG, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes. 1984; 33:825–31.

25. Kopman JA, Kim DM, Rahman SS, Arandia JA, Karimbux NY, Fiorellini JP. Modulating the effects of diabetes on osseointegration with aminoguanidine and doxycycline. J Periodontol. 2005; 76:614–20.

26. Hasegawa H, Ozawa S, Hashimoto K, Takeichi T, Ogawa T. Type 2 diabetes impairs implant osseointegration capacity in rats. Int J Oral Maxillofac Implants. 2008; 23:237–46.
27. Kwon PT, Rahman SS, Kim DM, Kopman JA, Karimbux NY, Fiorellini JP. Maintenance of osseointegration utilizing insulin therapy in a diabetic rat model. J Periodontol. 2005; 76:621–6.

28. Shirakura M, Fujii N, Ohnishi H, Taguchi Y, Ohshima H, Nomura S, et al. Tissue response to titanium implantation in the rat maxilla, with special reference to the effects of surface conditions on bone formation. Clin Oral Implants Res. 2003; 14:687–96.

29. Shyng YC, Devlin H, Ou KL. Bone formation around immediately placed oral implants in diabetic rats. Int J Prosthodont. 2006; 19:513–4.
Fig. 1.
Schematic design of the experiment A and titanium implants placed in the extraction socket of both maxillary first molars B.
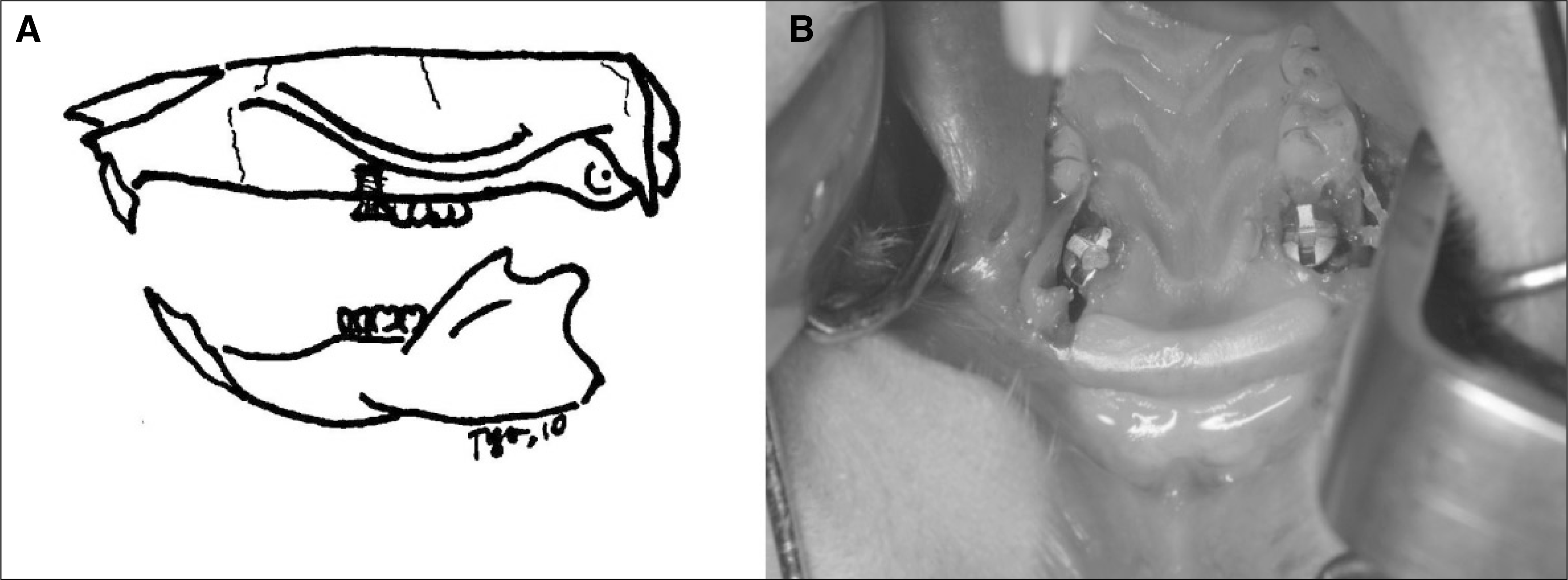
Fig. 2.
Hematoxylin and eosin-stained histological sections of bone implant interface 2 weeks after implantation.(original magnification ×40) No apparent differences between the groups could be observed. A. Control group. B. Diabetic group. C. Insulin-treated group.
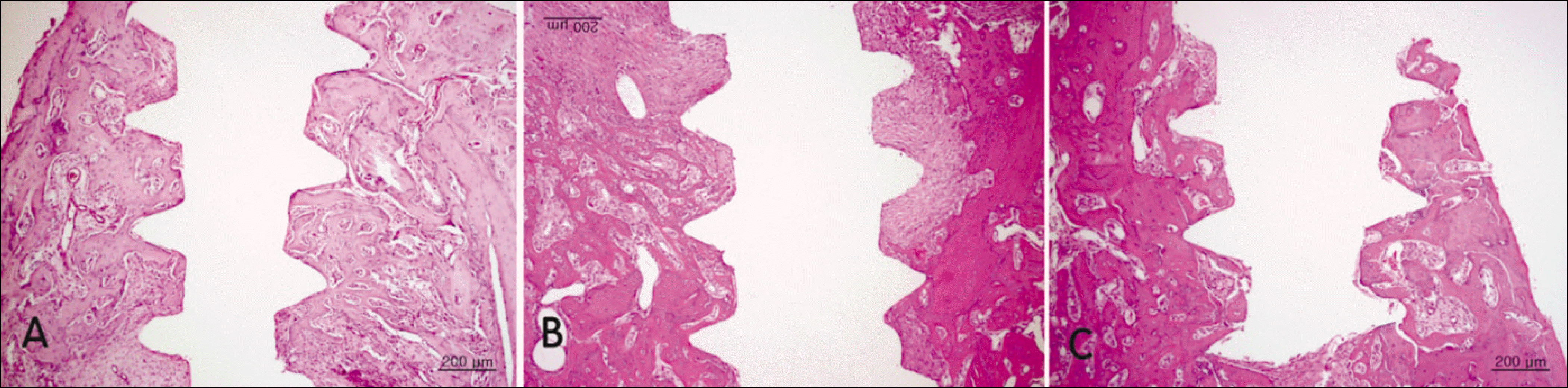
Fig. 3.
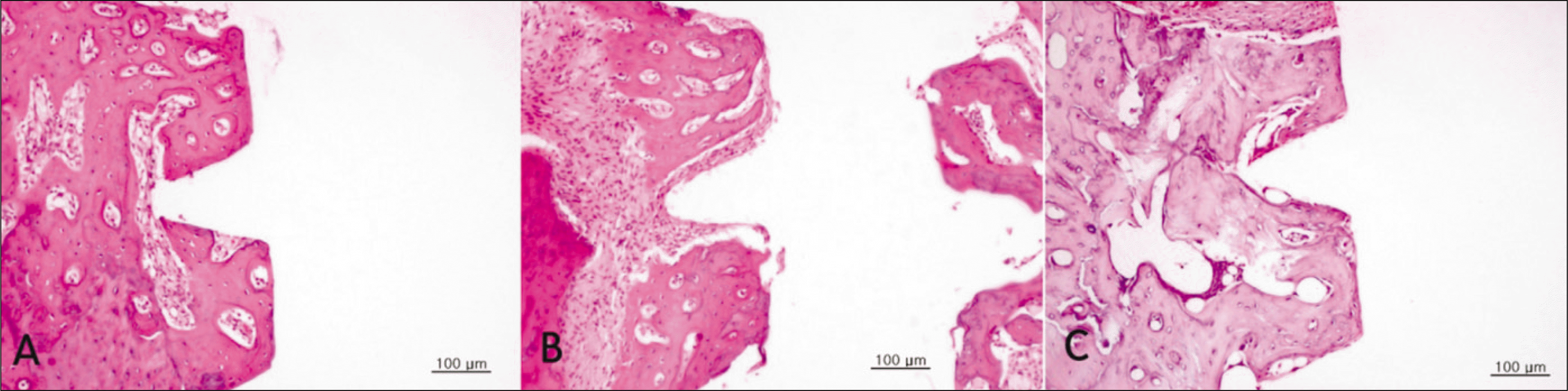
Hematoxylin and eosin-stained sections of bone implant interface 2 weeks after implantation.(original magnification ×200) Less organized and more cellular woven bone in sections from diabetic group is observed. A. Control group. B. Diabetic group. C. Insulin-treated group.
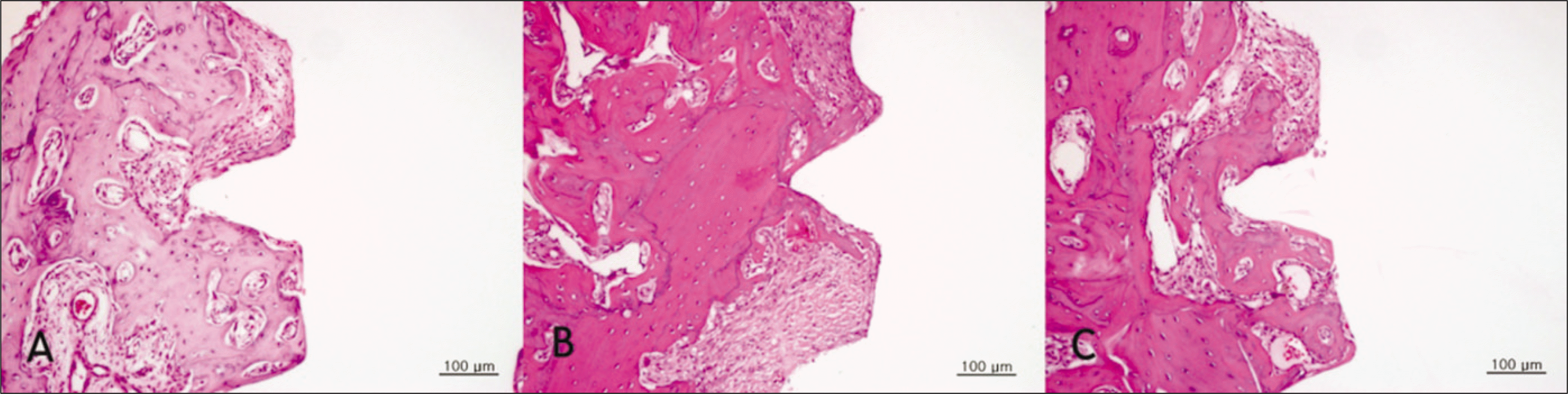
Fig. 4.
Hematoxylin and eosin-stained sections of bone implant interface 4 weeks after implantation.(original magnification ×40) Decreased bone apposition was observed in the diabetic group, which suggest an impaired osteoblastic function or mineralization defect. A. Control group. B. Diabetic group. C. Insulin-treated group.
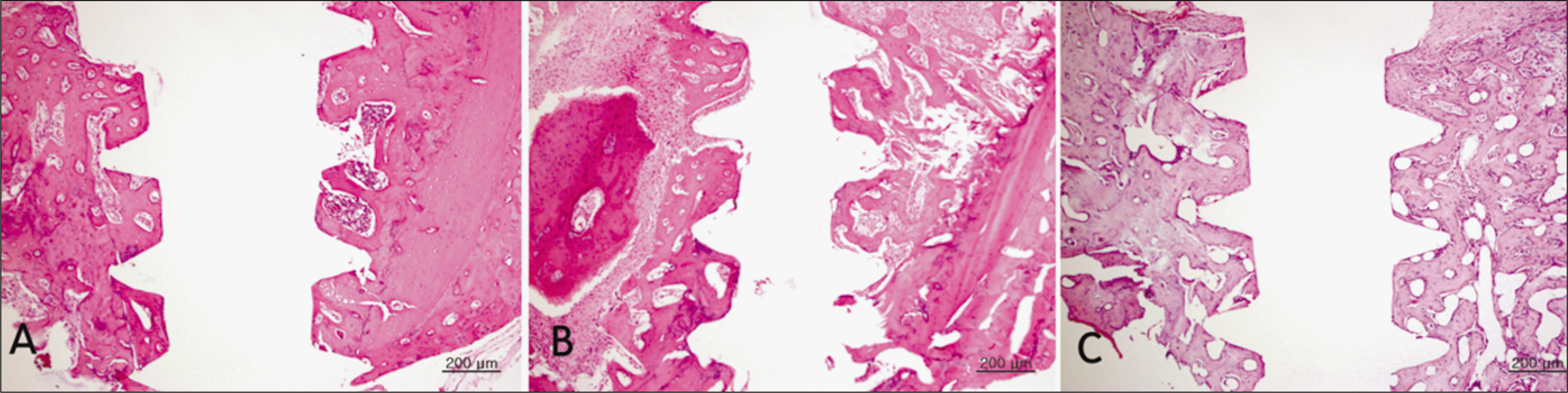
Fig. 5.
Hematoxylin and eosin-stained sections of bone implant interface 4 weeks after implantation.(original magnification ×200) Calcified mature woven bone with the marked restitution of marrow can be observed in the control and insulin-treated groups. A. Control group. B. Diabetic group. C. Insulin-treated group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download