Abstract
Complex human tissues harbor stem cells and precursor cells, which are responsible for tissue development or repair. Recently, dental tissues such as dental pulp, periodontal ligament (PDL), dental follicle have been identified as easily accessible sources of undifferentiated cells.
These tissues contain mesenchymal stem cells that can be differentiate into bone, cartilage, fat or muscle by exposing them to specific growth conditions. In this study, the authors procured the stem cell from pulp, PDL, and dental follicle and differentiate them into osteoblast and examine the bone induction capacity.
Dental pulp stem cell (DPSC), periodontal ligament stem cell (PDLSC), and dental follicle precursor cell (DFPC) were obtained from human 3rd molar and cultured. Each cell was analyzed for presence of stem cell by fluorescence activated cell sorter (FACs) against CD44, CD105 and CD34, CD45. Each stem cell was cultured, expanded and grown in an osteogenic culture medium to allow formation of a layer of extracellular bone matrix. Osteogenic pathway was checked by alizarin red staining, alkaline phosphatase (ALP) activity test and RT-PCR for ALP and osteocalcin (OCN) gene expression.
According to results from FACs, mesenchymal stem cell existed in pulp, PDL, and dental follicle. As culturing with bone differentiation medium, stem cells were differentiated to osteoblast like cell. Compare with stem cell from pulp, PDL and dental follicle-originated stem cell has more osteogenic effect and it was assumed that the character of donor cell was able to affect on differential potency of stem cell. From this article, we are able to verify the pulp, PDL, and dental follicle from extracted tooth, and these can be a source of osteoblast and stem cell for tissue engineering.
References
1. Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961; 14:213–22.

2. Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997; 276:71–4.

3. Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000; 3:537–44.

4. Khang GS, Lee SJ, Lee IW, Lee HB. Stem cell: scientific progress and future research direction. Polym Sci Technol. 2002; 13:4–14.
5. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–7.

6. National Institutes of Health (NIH). Stem cells: scientific progress and future research direction [internet]. Bethesda, MD: NIH;2001. Available from:. http://stemcells.nih.gov/staticre-sources/info/scireport/PDFs/fullrptstem.pdf.
7. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000; 97:13625–30.
8. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–55.

11. Guillot PV, Cui W, Fisk NM, Polak DJ. Stem cell differentiation and expansion for clinical applications of tissue engineering. J Cell Mol Med. 2007; 11:935–44.

12. Mathe G, Amiel JL, Schwarzenberg L, Cattan A, Schneider M. Haematopoietic chimera in man after allogenic (homologous) bone-marrow transplantation. (Control of the secondary syndrome. Specific tolerance due to the chimerism). Br Med J. 1963; 28:1633–5.
13. Tropel P, Noe ¨l D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004; 295:395–406.

14. Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002; 73:1919–25.

15. Kunter U, Rong S, Djuric Z, Boor P, Mu ¨ller-Newen G, Yu D, et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006; 17:2202–12.

16. Zhang H, Huang Z, Xu Y, Zhang S. Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol Res. 2006; 28:104–12.

17. Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001; 97:1227–31.

18. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999; 5:309–13.

19. Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002; 99:8932–7.

20. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987; 20:263–72.

21. Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, et al. Stem cells associated with macroporous bioce-ramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007; 13:947–55.

22. Schimming R, Schmelzeisen R. Tissue-engineered bone for maxillary sinus augmentation. J Oral Maxillofac Surg. 2004; 62:724–9.

23. Pradel W, Eckelt U, Lauer G. Bone regeneration after enucleation of mandibular cysts: comparing autogenous grafts from tissue-engineered bone and iliac bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:285–90.

24. Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop. 2007; 27:629–34.

25. Morsczeck C, Schmalz G, Reichert TE, Vo ¨llner F, Galler K, Driemel O. Somatic stem cells for regenerative dentistry. Clin Oral Investig. 2008; 12:113–8.

26. Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006; 184:105–16.

27. Trubiani O, Orsini G, Zini N, Di Iorio D, Piccirilli M, Piattelli A, et al. Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: a morphological report. J Biomed Mater Res A. 2008; 87:986–93.

28. Morsczeck C, Moehl C, Go ¨tz W, Heredia A, Scha ¨ffer TE, Eckstein N, et al. In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol Int. 2005; 29:567–75.

29. Zhang W, Walboomers XF, van Kuppevelt TH, Daamen WF, van Damme PA, Bian Z, et al. In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. J Tissue Eng Regen Med. 2008; 2:117–25.

30. Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990; 143:420–30.

31. Kotobuki N, Hirose M, Funaoka H, Ohgushi H. Enhancement of in vitro osteoblastic potential after selective sorting of osteoblasts with high alkaline phosphatase activity from human osteoblast-like cells. Cell Transplant. 2004; 13:377–83.

32. Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008; 29:3053–61.

33. Laino G, Carinci F, Graziano A, d’ Aquino R, Lanza V, De Rosa A, et al. In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg. 2006; 17:511–5.

34. Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001; 29:532–9.

35. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002; 81:531–5.

36. Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003; 82:976–81.

37. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003; 100:5807–12.

Fig. 1.
Morphology of stem cells from dental tissues.(2-3 passages)
A, B: DPSC (x 200, x 100), C, D: PDLSC (x 200, x 100), E, F: DFPC (x 200, x 100).
(DPSC: dental pulp stem cell, PDLSC: periodontal ligament stem cell, DFPS: dental follicle precursor cell)
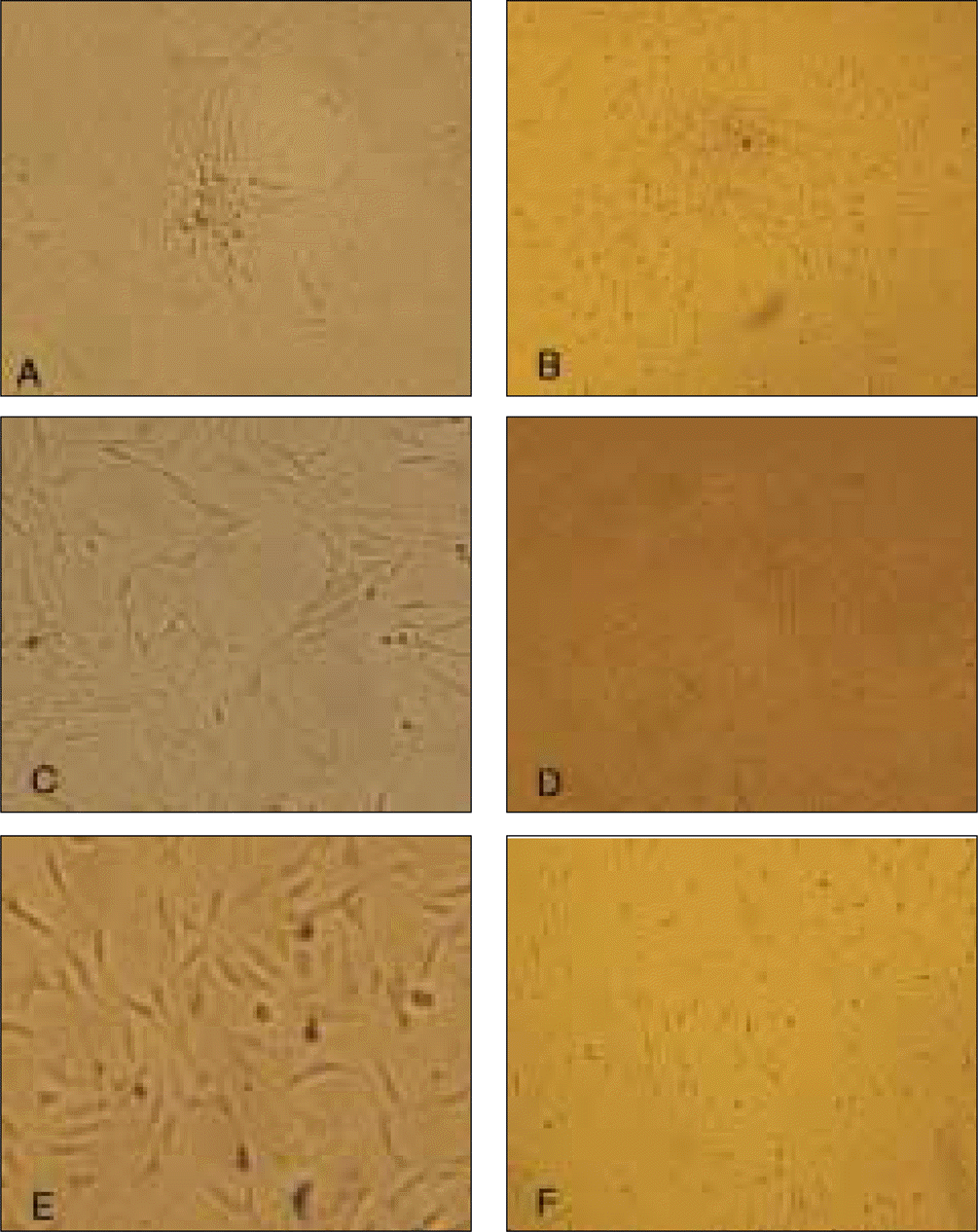
Fig. 2.
Characterization of DPSC, PDLSC, and DFPC immunophenotype in vitro. (DPSC: dental pulp stem cell, PDLSC: periodontal ligament stem cell, DFPS: dental follicle precursor cell)
Fig. 3.
Morphology of cells in osteogenic culture media after 21 days (x 100).
A, C, E: DPSC, PDLSC, DFPC. In universal culture media after 21 days (X 100). B, D, F: DPSC, PDLSC, DFPC.
(DPSC: dental pulp stem cell, PDLSC: periodontal ligament stem cell, DFPS: dental follicle precursor cell)
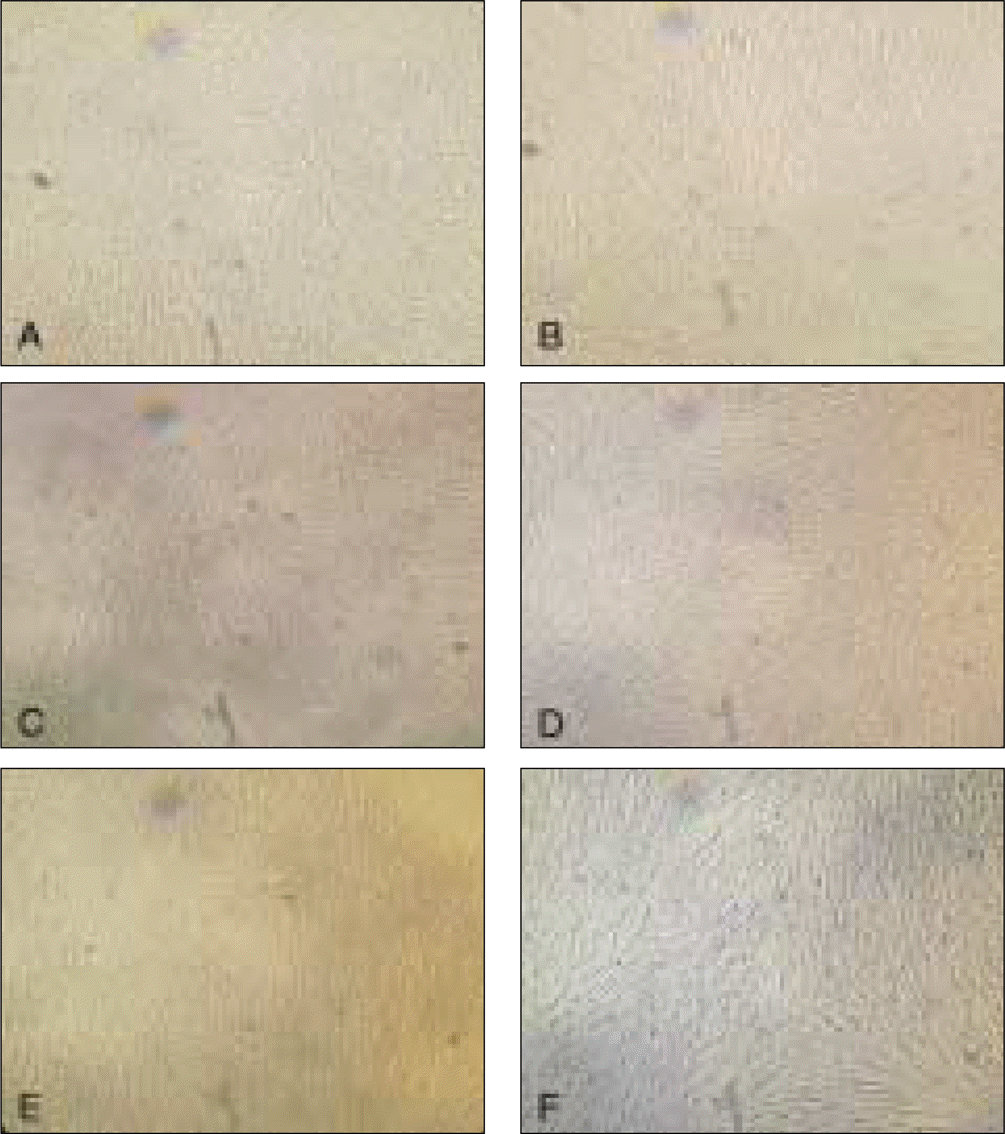
Fig. 4.
Alizarin red staining of cultured cells in osteogenic media after 21 days.
A, C, E: DPSC, PDLSC, DFPC. In universal culture media. B, D, F: DPSC, PDLSC, DFPC.
(DPSC: dental pulp stem cell, PDLSC: periodontal ligament stem cell, DFPS: dental follicle precursor cell)
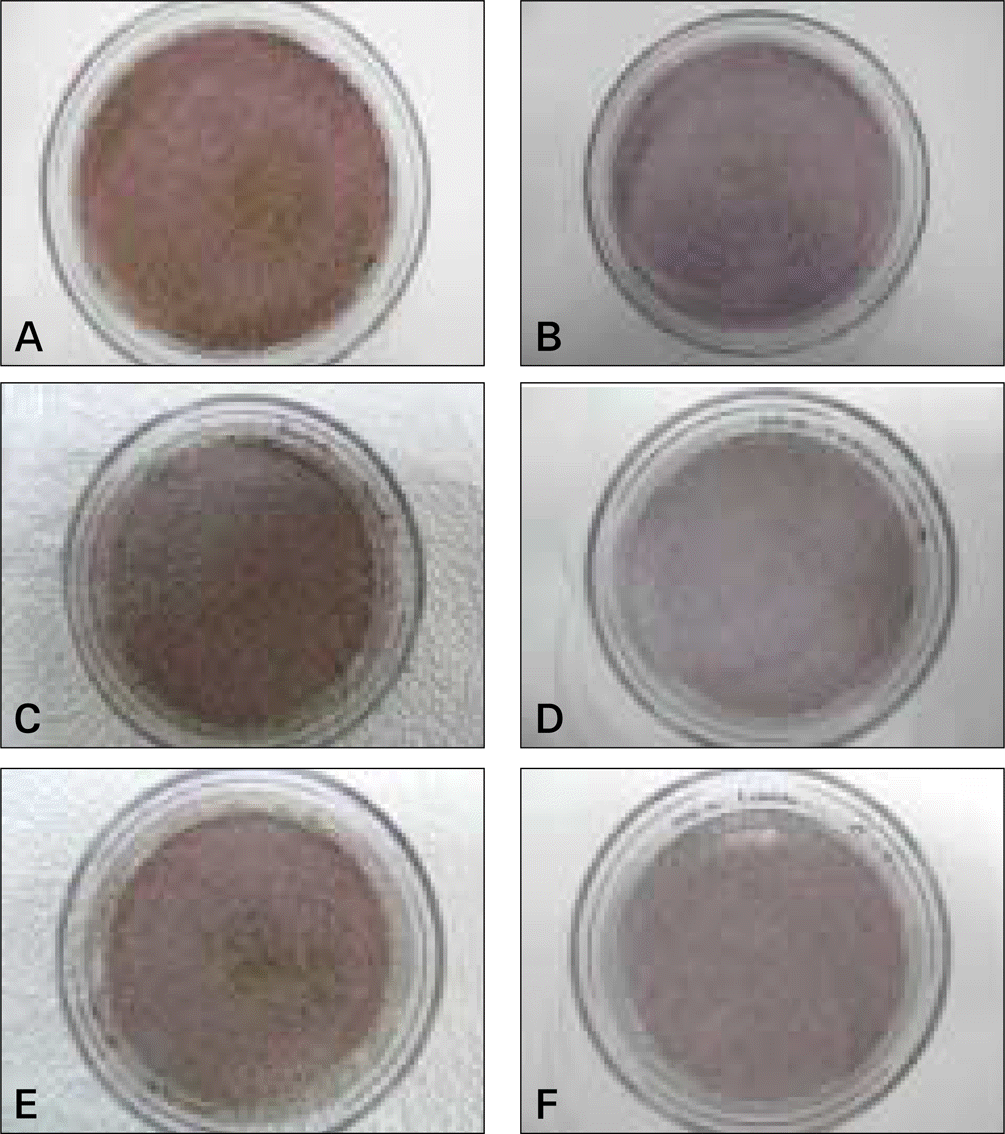
Fig. 5.
ALP activity test after 7, 14, 21 days of culture. A: Experimental group (osteogenic media), B: Control group (universal media).
(DPSC: dental pulp stem cell, PDLSC: periodontal ligament stem cell, DFPS: dental follicle precursor cell)
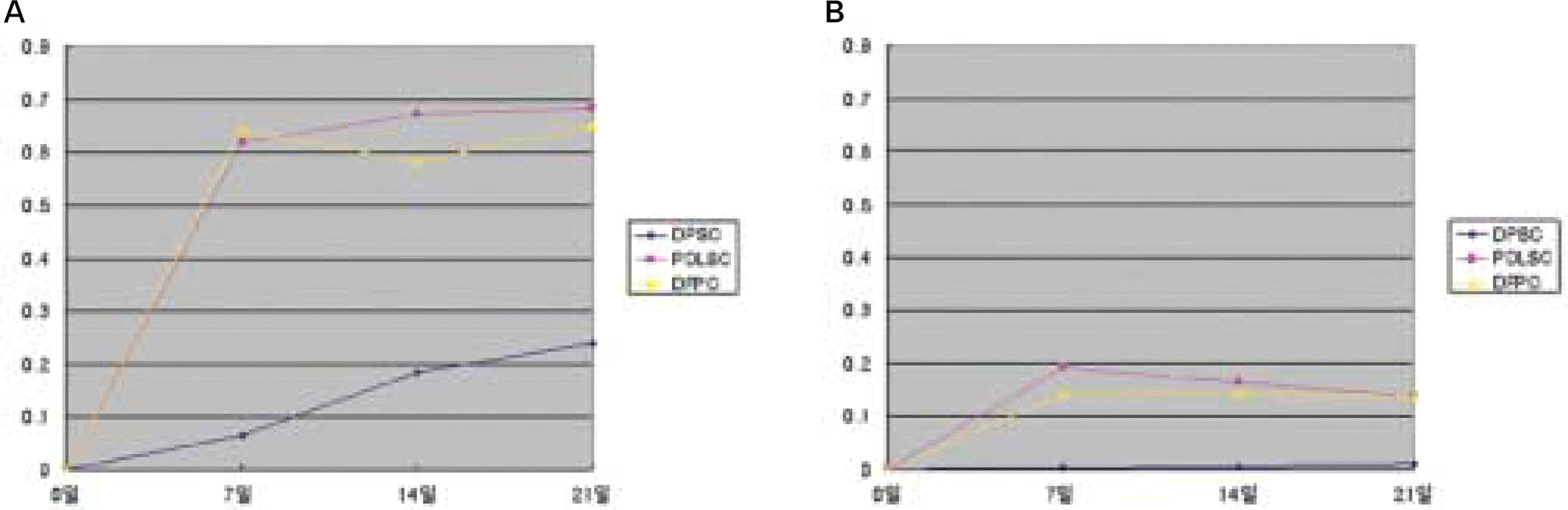
Fig. 6.
RT-PCR analysis for expression of osteoblast markers after 7, 14, 21 days of culture (exp: osteogenic media, control: universal media). A: ALP, B: OCN.
(ALP: alkaline phosphatase, OCN: osteocalcin, DPSC: dental pulp stem cell, PDLSC: periodontal ligament stem cell, DFPS: dental follicle precursor cell, GAPDH: glyceraldehyde 3-phosphate dehydrogenase)
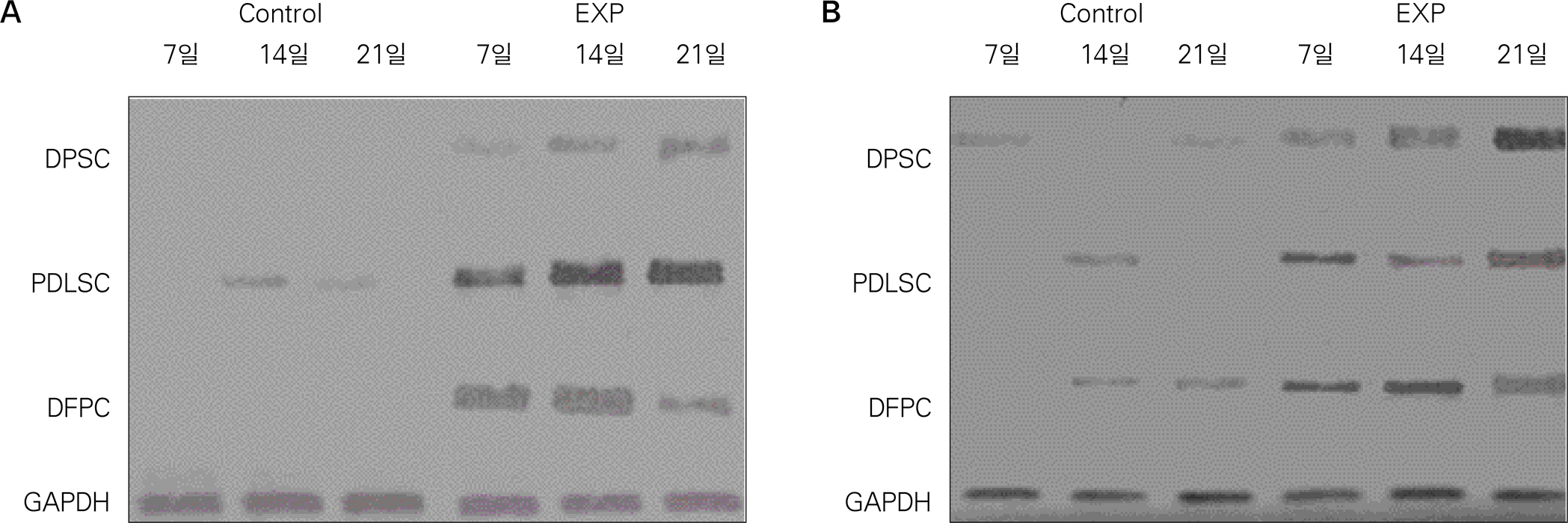
Table 1.
Characteristics of samples. Sample number, sex and age, location of extracted teeth, and remark.
Table 2.
RT-PCR primer list and sequence.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download