Abstract
Introduction
There are few reports on tooth eruption through Bio-Oss grafts. To our knowledge, there are no reports on whether teeth can erupt normally through the grafts. The aim of this study was to examine the effect of Bio-Oss grafts on tooth eruption in a canine model.
Materials and Methods
In five 10-week-old dogs, the deciduous third mandibular molars in one jaw quadrant of each animal were extracted and the fresh extraction sockets were then filled with Bio-Oss particles (experimental side). No such treatments were performed on the contralateral side (control side). A clinical and radiological evaluation was carried out every other week to evaluate the eruption level of the permanent third mandibular premolars and compare the eruption levels between the two sides.
Results
At week 4 after the experiment, the permanent third premolars began to erupt on both sides. At week 12, the crown of the permanent third premolar emerged from the gingiva on both sides. At week 20, the permanent third premolars on both sides erupted enough to occlude the opposing teeth. No significant differences were found between the control and experimental sides in terms of the eruption speed of the permanent third molars.
Go to : 
REFERENCES
1. Freihofer HP, Borstlap WA, Kuijpers-Jagtman AM, Voorsmit RA, van Damme PA, Heidb¨chel KL, et al. Timing and trans-plant materials for closure of alveolar clefts. A clinical comparison of 296 cases. J Craniomaxillofac Surg. 1993; 21:143–8.
2. Witsenburg B, Remmelink HJ. Reconstruction of residual alveo-lo-palatal bone defects in cleft patients. A retrospective study. J Craniomaxillofac Surg. 1993; 21:239–44.
3. Kalaaji A, Lilja J, Friede H, Elander A. Bone grafting in the mixed and permanent dentition in cleft lip and palatepatients: longterm results and the role of the surgeon’ s experience. J Craniomaxillofac Surg. 1996; 24:29–35.
4. Diès F, Etienne D, Abboud NB, Ouhayoun JP. Bone regeneration in extraction sites after immediate placement of an e-PTFE membrane with or without a biomaterial. A report on 12 consecutive cases. Clin Oral Implants Res. 1996; 7:277–85.

5. Merkx MA, Maltha JC, van’ t Hoff M, Kuijpers-Jagtman AM, Freihofer HP. Tooth eruption through autogenous and xenogenous bone transplants: a histological and radiographic evaluation in beagle dogs. J Craniomaxillofac Surg. 1997; 25:212–9.

6. Becker W, Clokie C, Sennerby L, Urist MR, Becker BE. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: case reports. J Periodontol. 1998; 69:414–21.

7. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets: 2. Histochemical observations at 9 months. J Periodontol. 2001; 72:152–9.

8. Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res. 2003; 14:137–43.

9. Froum S, Cho SC, Elian N, Rosenberg E, Rohrer M, Tarnow D. Extraction sockets and implantation of hydroxyapatites with membrane barriers: a histologic study. Implant Dent. 2004; 13:153–64.

10. Nevins M, Camelo M, de Paoli S, Friedland B, Schenk RK, Parma-Benfenati S, et al. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006; 26:19–29.

11. Norton MR, Odell EW, Thompson ID, Cook RJ. Efficacy of bovine bone mineral for alveolar augmentation: a human histologic study. Clin Oral Implants Res. 2003; 14:775–83.

12. Esposito M, Grusovin MG, Coulthard P, Worthington HV. The efficacy of various bone augmentation procedures for dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2006; 21:696–710.
13. Nemcovsky CE, Artzi Z, Moses O, Gelernter I. Healing of marginal defects at implants placed in fresh extraction sockets or after 4-6 weeks of healing. A comparative study. Clin Oral Implants Res. 2002; 13:410–9.
14. Zins JE, Whitaker LA. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 1983; 72:778–85.
15. Koole R, Bosker H, van der Dussen FN. Late secondary autogenous bone grafting in cleft patients comparing mandibular (ec-tomesenchymal) and iliac crest (mesenchymal) grafts. J Craniomaxillofac Surg. 1989; 17(Suppl 1):28–30.

16. Sugimoto A, Ohno K, Michi K, Kanegae H, Aigase S, Tachikawa T. Effect of calcium phosphate ceramic particle insertion on tooth eruption. Oral Surg Oral Med Oral Pathol. 1993; 76:141–8.

17. Cardaropoli G, Araújo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced - augmented and non-augmented - defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005; 32:435–40.

18. Klinge B, Alberius P, Isaksson S, Jonssen J. Osseous response to implanted natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J Oral Maxillofac Surg. 1992; 50:241–9.

19. Heberer S, Al-Chawaf B, Hildebrand D, Nelsen JJ, Nelson K. Histomorphometric analysis of extraction sockets augmented with Bio-Oss Collagen after a 6-week healing period: a prospective study. Clin Oral Implants Res. 2008; 19:1219–25.

20. Pilipili CM, Goret-Nicaise M, Dhem A. Microradiographic aspects of the growing mandibular body during permanent premolar eruption in the dog. Eur J Oral Sci. 1998; 106(Suppl 1):429–36.

21. Larson EK, Cahill DR, Gorski JP, Marks SC Jr. The effect of removing the true dental follicle on premolar eruption in the dog. Arch Oral Biol. 1994; 39:271–5.

22. Hooft J, Mattheeuws D, van Bree P. Radiology of deciduous teeth resorption and definitive teeth eruption in the dog. J Small Anim Pract. 1979; 20:175–80.

23. Cahill DR. The histology and rate of tooth eruption with and without temporary impaction in the dog. Anat Rec. 1970; 166:225–37.

24. Hyun HK, Lee SJ, Lee SH, Hahn SH, Kim JW. Clinical characteristics and complications associated with mesiodentes. J Oral Maxillofac Surg. 2009; 67:2639–43.

25. Jacobs SG. The impacted maxillary canine. Further observations on aetiology, radiographic localization, prevention/interception of impaction, and when to suspect impaction. Aust Dent J. 1996; 41:310–6.

26. Prabhu NT, Rebecca J, Munshi AK. Dentigerous cyst with inflammatory etiology from a deciduous predecessor-report of a case. J Indian Soc Pedod Prev Dent. 1996; 14:49–51.
27. Maynard JG Jr, Ochsenbein C. Mucogingival problems, prevalence and therapy in children. J Periodontol. 1975; 46:543–52.

28. Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003; 30:809–18.
29. Araújo MG, Carmagnola D, Berglundh T, Thilander B, Lindhe J. Orthodontic movement in bone defects augmented with Bio-Oss. An experimental study in dogs. J Clin Periodontology. 2001; 28:73–80.
Go to : 
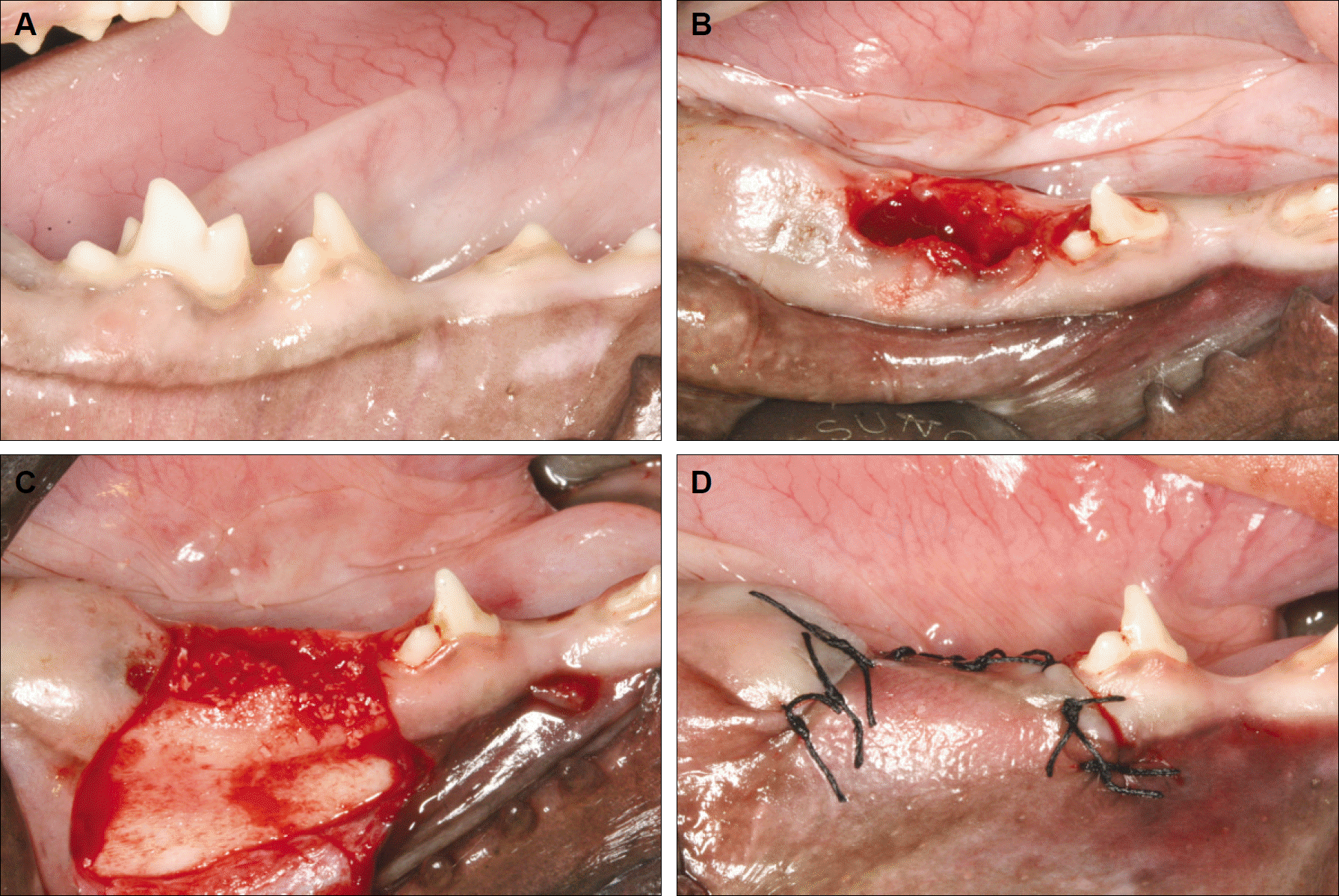 | Fig. 1.A. Clinical features before treatment. B. Clinical features after extraction of the primary 3rd molar. C. Clinical features after filling the extraction sockets with Bio-Oss particles. D. Clinical features after treatment. |
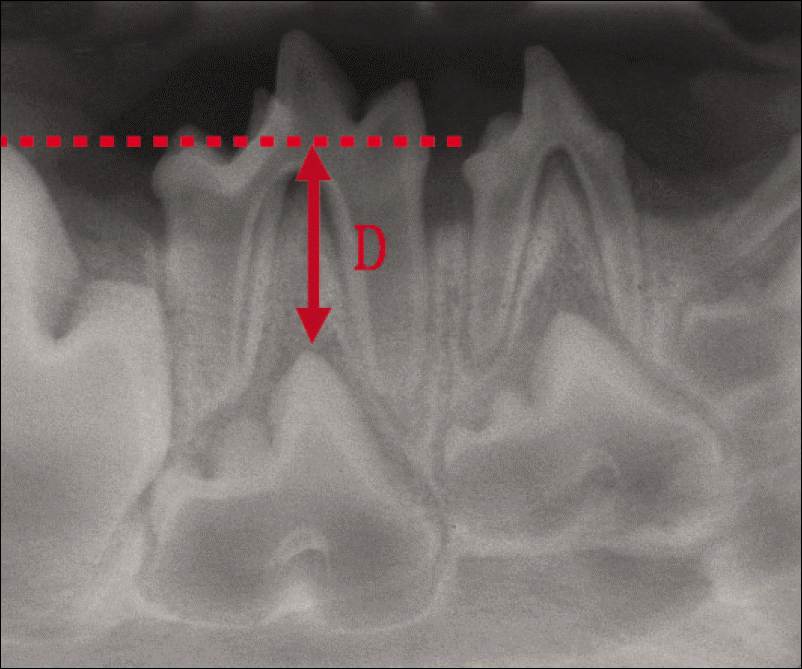 | Fig. 2.Measurements of tooth eruption levels made on periapical radiograph. D: tooth eruption level |
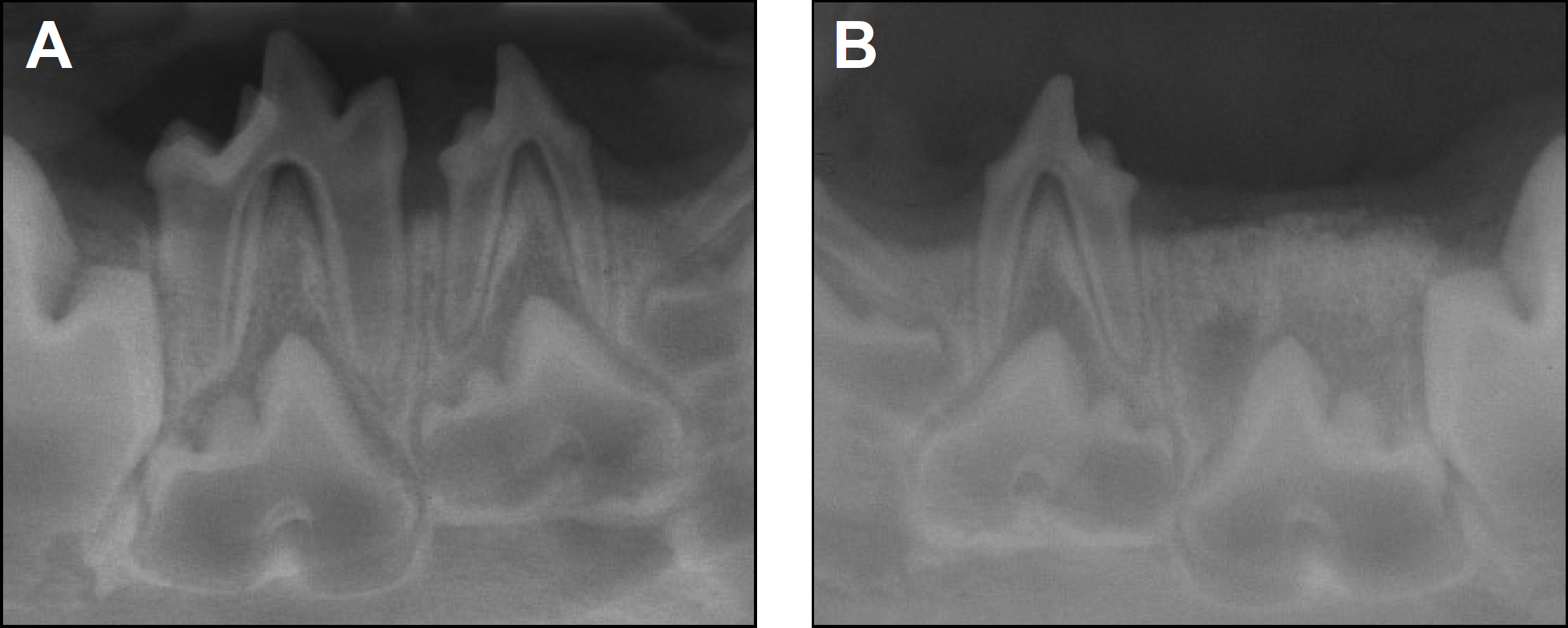 | Fig. 3.Periapical radiographs taken 4 weeks after treatment. A. Control side. B. Experimental side. |
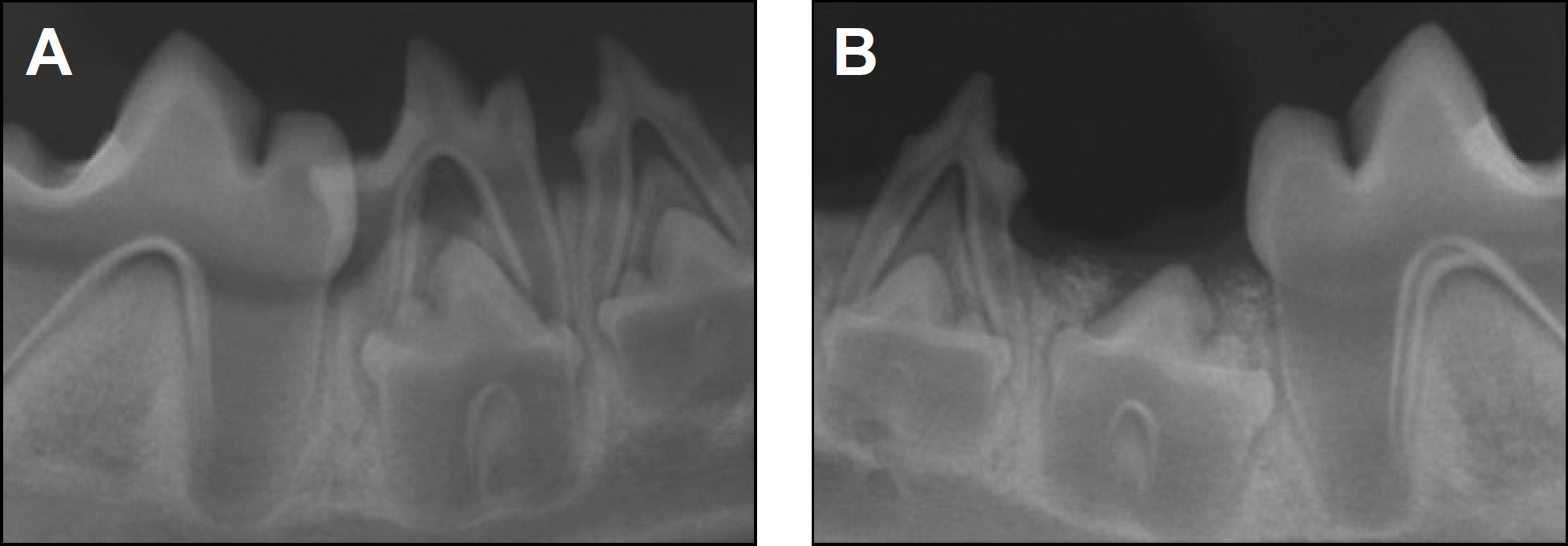 | Fig. 4.Periapical radiographs taken 8 weeks after treatment. A. Control side. B. Experimental side. |
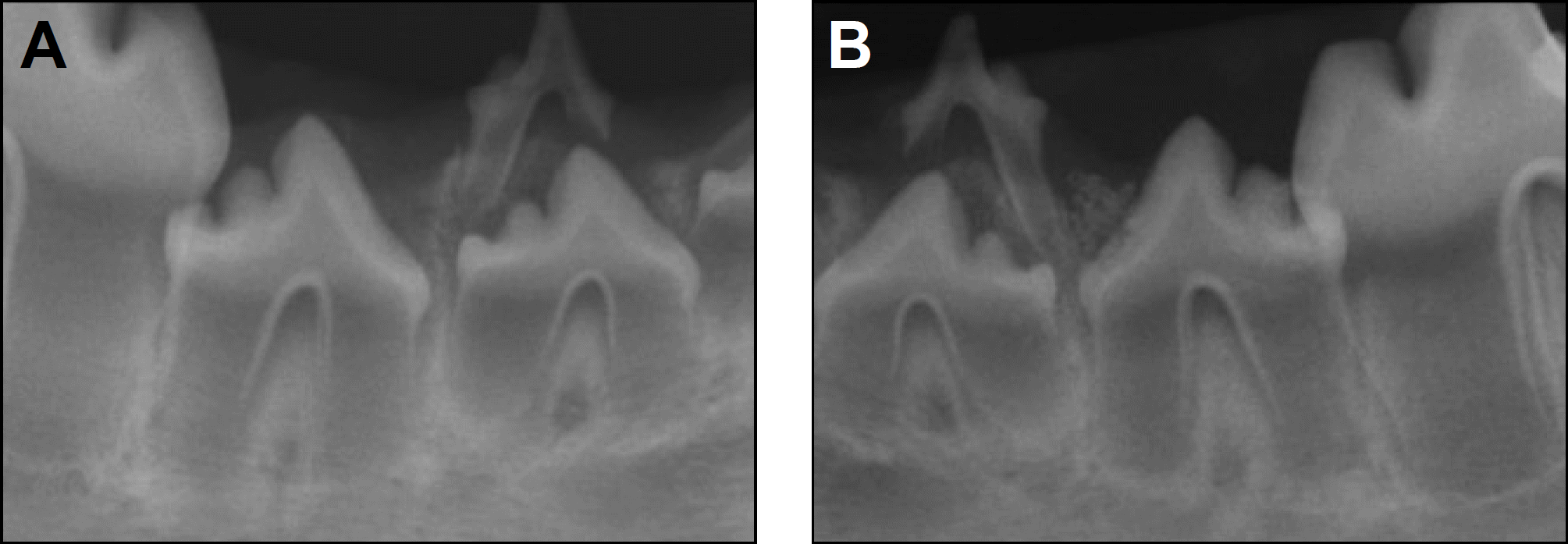 | Fig. 5.Periapical radiographs taken 12 weeks after treatment. A. Control side. B. Experimental side. |
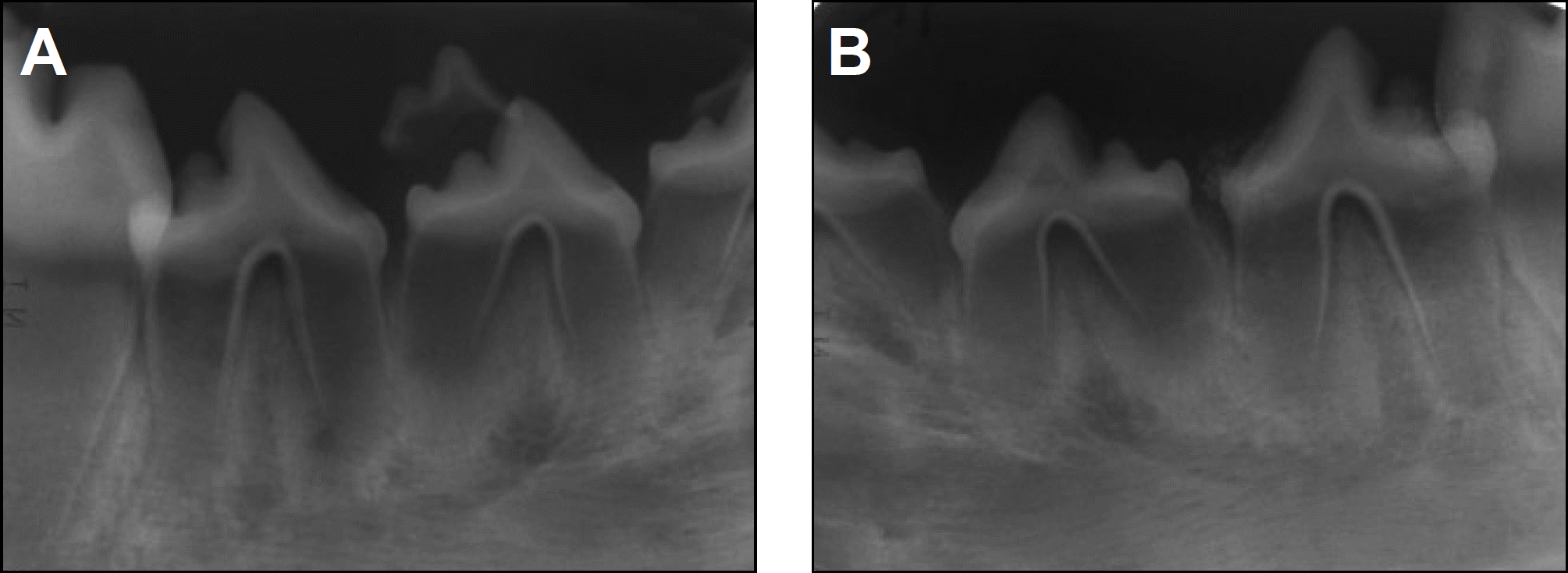 | Fig. 6.Periapical radiographs taken 16 weeks after treatment. A. Control side. B. Experimental side. |
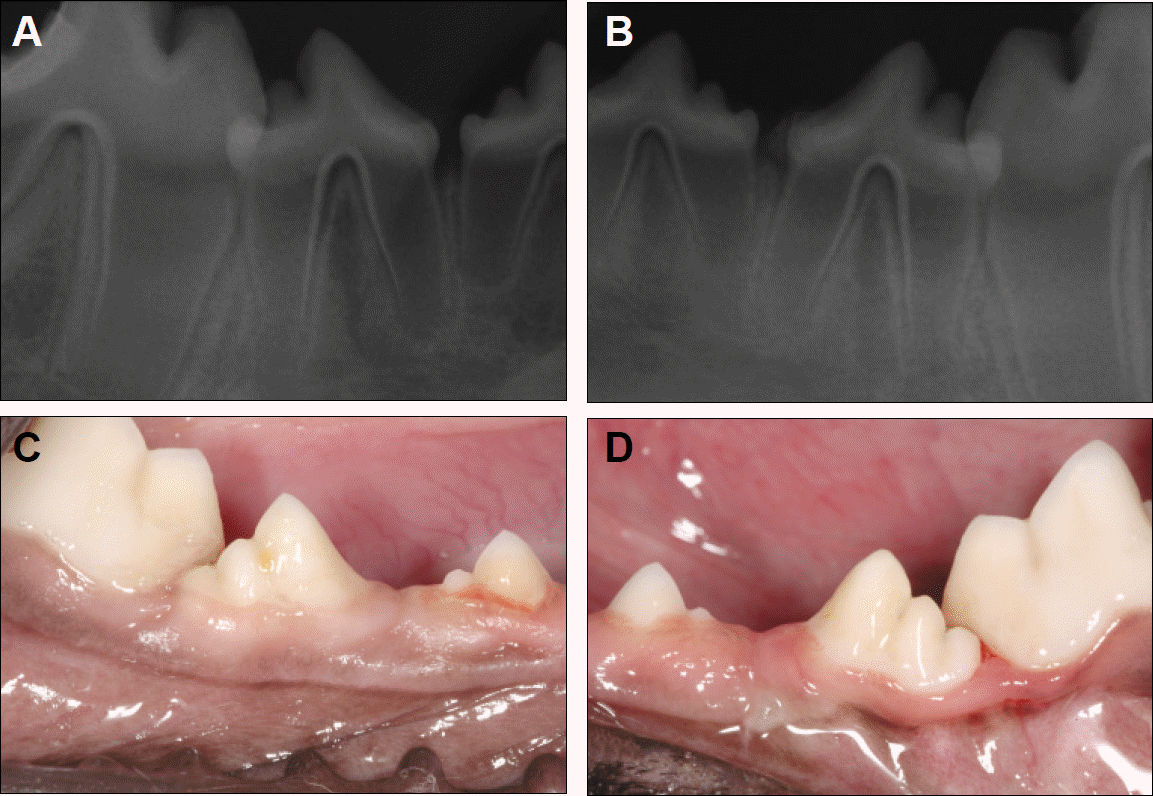 | Fig. 7.Periapical radiographs and clinical features taken 20 weeks after treatment. A, C. Control side. B, D. Experimental side. |
Table 1.
Results from measurements describing tooth eruption levels during the time of eruption on the control and experimental sides




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download