Abstract
Introduction
This study examined the genetic influence of mandibular prognathism epidemiologically in Korean families.
Materials and Methods
Over a 5-year period from 2005 to 2009, a questionnaire with a pedigree chart was given to 100 (male 51, female 49) probands with skeletal Class III mandibular prognathism, who had undergone orthognathic surgery in Samsung Medical Center.
Results
The average age of the probands was 22.1. The average SNA, SNB and ANB angles of the probands were 81.2�, 84.1� and -2.9�, respectively. A total of 2729 (male 1,354, female 1,375) family members were examined, and the affected ratio of the families was 3.5% with no significant difference between genders. 45% of families had at least one member with a Class III malocclusion other than the proband. The affected ratio of the first-degree relatives (10.9%) was significantly higher than those of the second-degree (3.3%) and third-degree (1.9%) relatives. The affected ratio of the total relatives from the male probands (4.2%) was significantly higher than that of the female probands (2.8%). Heritability (h2, Falconer’ method) was estimated to be 29.8% (0.298±0.059) in first-degree relatives.
Go to : 
REFERENCES
1. Nakasima A, Ichinose M, Nakata S. Genetic and environmental factors in the development of so-called pseudo- and true mesioc-clusions. Am J Orthod Dentofacial Orthop. 1986; 90:106–16.
2. Litton SF, Ackermann LV, Isaacson RJ, Shapiro BL. A genetic study of Class 3 malocclusion. Am J Orthod. 1970; 58:565–77.
3. Newman GV. Prevalence of malocclusion in children six to fourteen years of age and treatment in preventable cases. J Am Dent Assoc. 1956; 52:566–75.

4. Emrich RE, Brodie AG, Blayney JR. Prevalence of Class 1, Class 2, and Class 3 malocclusions (Angle) in an urban population. An epidemiological study. J Dent Res. 1965; 44:947–53.
5. Proffit WR, Fields HW Jr., Moray LJ. Prevalence of malocclusion and orthodontic treatment need in the United States: estimates from the NHANES III survey. Int J Adult Orthodon Orthognath Surg. 1998; 13:97–106.
6. Garner LD, Butt MH. Malocclusion in black Americans and Nyeri Kenyans. An epidemiologic study. Angle Orthod. 1985; 55:139–46.
7. Ishii H, Morita S, Takeuchi Y, Nakamura S. Treatment effect of combined maxillary protraction and chincap appliance in severe skeletal Class III cases. Am J Orthod Dentofacial Orthop. 1987; 92:304–12.

8. Kang HK, Ryu YK. A study on the prevalence of malocclusion of Yonsei university students in 1991. Korean J Orthod. 1992; 22:691–701.
9. Yoo YK, Kim NI, Lee HK. A study on the prevalence of malocclusion in 2,378 Yonsei university students. Korean J Orthod. 1971; 2:35–40.
10. Pascoe JJ, Hayward JR, Costich ER. Mandibular prognathism: its etiology and a classification. J Oral Surg Anesth Hosp Dent Serv. 1960; 18:21–4.
11. Gold JK. A new approach to the treatment of mandibular prognathism. Am J Orthod. 1949; 35:893–912. illust.

12. Watanabe M, Suda N, Ohyama K. Mandibular prognathism in Japanese families ascertained through orthognathically treated patients. Am J Orthod Dentofacial Orthop. 2005; 128:466–70.

13. Wolff G, Wienker TF, Sander H. On the genetics of mandibular prognathism: analysis of large European noble families. J Med Genet. 1993; 30:112–6.

14. El-Gheriani AA, Maher BS, El-Gheriani AS, Sciote JJ, Abu-Shahba FA, Al-Azemi R, et al. Segregation analysis of mandibular prognathism in Libya. J Dent Res. 2003; 82:523–7.

15. Cruz RM, Krieger H, Ferreira R, Mah J, Hartsfield J Jr, Oliveira S. Major gene and multifactorial inheritance of mandibular prognathism. Am J Med Genet A. 2008; 146A:71–7.

16. Falconer DS, MacKay TFC. Introduction to quantitative genetics. 4th ed.Essex, England: Longman;1996.
17. Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965; 29:51–76.

18. Lee CH, Lee SH, Kim HS, Kwon TG. Analysis of familial tendency in skeletal class III malocclusion. J Korean Assoc Oral Maxillofac Surg. 2006; 32:506–13.
19. Jacobson A, Evans WG, Preston CB, Sadowsky PL. Mandibular prognathism. Am J Orthod. 1974; 66:140–71.

21. Oh J, Wang CJ, Poole M, Kim E, Davis RC, Nishimura I, et al. A genome segment on mouse chromosome 12 determines maxillary growth. J Dent Res. 2007; 86:1203–6.

22. Dohmoto A, Shimizu K, Asada Y, Maeda T. Quantitative trait loci on chromosomes 10 and 11 influencing mandible size of SMXA RI mouse strains. J Dent Res. 2002; 81:501–4.

23. Yamaguchi T, Park SB, Narita A, Maki K, Inoue I. Genome-wide linkage analysis of mandibular prognathism in Korean and Japanese patients. J Dent Res. 2005; 84:255–9.

24. Frazier-Bowers S, Rincon-Rodriguez R, Zhou J, Alexander K, Lange E. Evidence of linkage in a Hispanic cohort with a Class III dentofacial phenotype. J Dent Res. 2009; 88:56–60.
25. Bui C, King T, Proffit W, Frazier-Bowers S. Phenotypic characterization of Class III patients. Angle Orthod. 2006; 76:564–9.
Go to : 
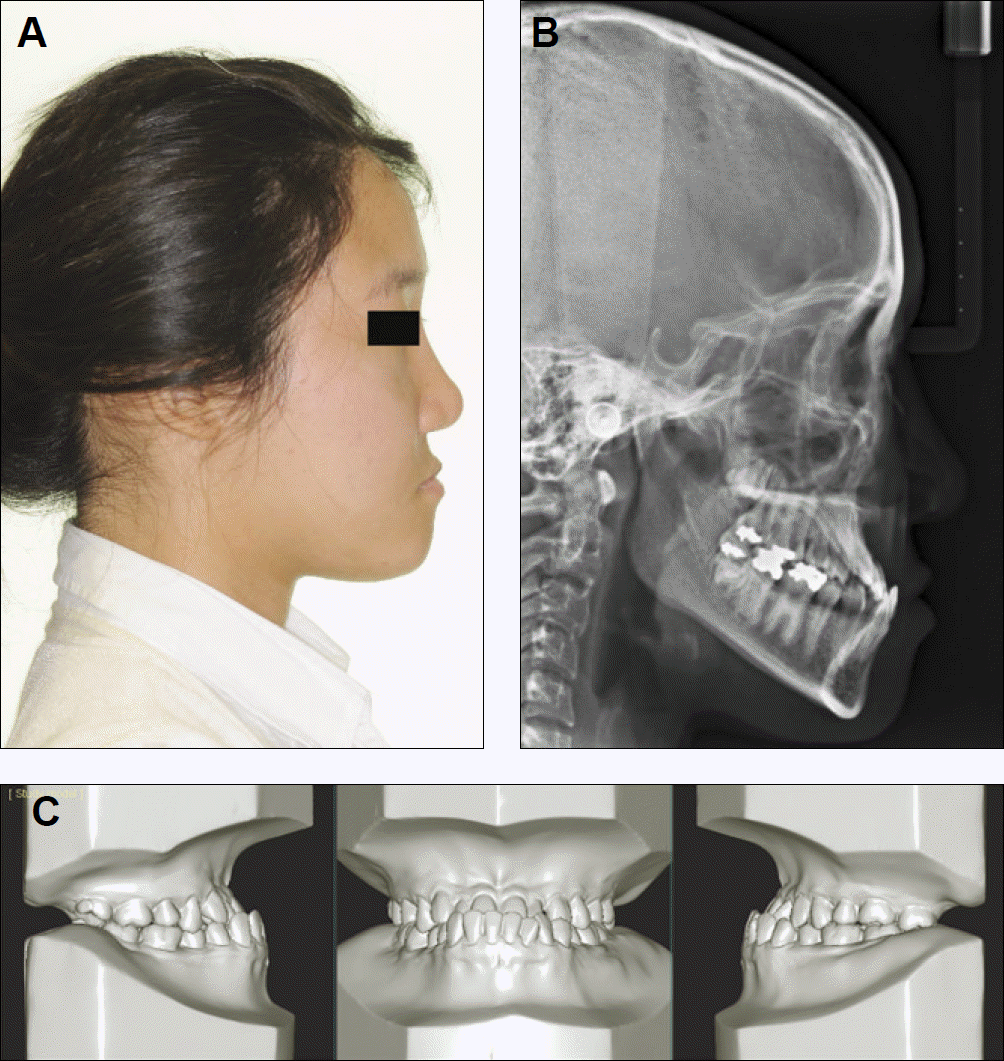 | Fig. 2.Diagnostic tools for the evaluation of skeletal Class III mandibular prognathism. A: Lateral photograph, B: Cephalogram, C: Study model. |
Table 1.
Cephalometric measurements of 100 probands
Table 2.
Affected ratios of relatives of 100 probands
| Relatives | Affected | Unaffected | Total | Affected ratio (%) | P1 |
|---|---|---|---|---|---|
| Male | 45 | 1,309 | 1,354 | 3.3 | |
| Female | 50 | 1,325 | 1,375 | 3.6 | 0.656 |
| Total | 95 | 2,634 | 2,729 | 3.5 |
Table 3.
Probands with at least one affected member
| Probands | Affected | Unaffected | Total | P1 |
|---|---|---|---|---|
| Male | 22 | 29 | 51 | |
| Female | 23 | 26 | 49 | 0.703 |
| Total | 45 (45%) | 55 (55%) | 100 (100%) |
Table 4.
Affected ratio of first-, second-, and third-degree relatives
(P2: x2 test to evaluate that the affected ratios were different among the 1st, 2nd and 3rd degree relatives. The affected ratio of the 1st degree relative was significantly different from that of the 2nd degree relative or that of the 3rd degree relative, respectively P<0.0001 by Fisher' s exact test with adjustments by permutation resampling)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download