Abstract
Introduction
TLR-5, a member of the toll-like receptor (TLR) family, is a element of the type I transmembrane receptors, which are characterized by an intracellular signaling domain homolog to the interleukin-1 receptor. These receptors recognize microbial components, particularly bacterial flagellin. All-trans retinoic acid (atRA, tretinoin), a natural metabolite of vitamin A, acts as a growth and differentiation factor in many tissues, and is also needed for immune functions. In this study, THP-1 human macrophage-monocytes were used to examine the mechanisms by which atRA regulated the expression of TLR-5. Because the molecular mechanism underlying this regulation at the transcriptional level is also unclear, this study examined which putative transcription factors are responsible for TLR-5 expression by atRA in immune cells.
Materials and Methods
This study examined whether atRA induces the expression of TLR-5 in THP-1 cells using reverse transcription-polymerase chain reaction (RT-PCR), and which transcription factors are involved in regulating the TLR-5 promoter in RAW264.7 cells using a reporter assay system. Western blot analysis was used to determine which signal pathway is involved in the expression of TLR-5 in atRA-treated THP-1 cells.
Results
atRA at a concentration of 10 nM greatly induced the expression of TLR-5 in THP-1 cells. Human TLR-5 promoter contains three Sp-1/GC binding sites around -50 bp and two NF-kB binding sites at -380 bp and -160 bp from the transcriptional start site of the TLR-5 gene. Sp-1/GC is primarily responsible for the constitutive TLR-5 expression, and may also contribute to NF-kB at -160 bp to induce TLR-5 after atRA stimulation in THP-1 cells. The role of NF-kB in TLR-5 expression was further confirmed by inhibitor pyrrolidine dithiocarbamate (PDTC) experiments, which greatly reduced the TLR-5 transcription by 70-80%.
Conclusion
atRA induces the expression of the human TLR-5 gene and NF-kB is a critical transcription factor for the atRA-induced expression of TLR-5. Accordingly, it is conceivable that retinoids are required for adequate innate and adaptive immune responses to agents of infectious diseases. atRA and various synthetic retinoids have been used therapeutically in human diseases, such as leukemia and other cancers due to the antiproliferative and apoptosis inducing effects of retinoids. Therefore, understanding the molecular regulatory mechanism of TLR-5 may assist in the design of alternative strategies for the treatment of infectious diseases, leukemia and cancers.
Go to : 
REFERENCES
1. Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988; 52:269–79.

2. Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997; 388:394–7.

3. Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006; 117:979–87. quiz 988.

4. Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy. 2002; 1:181–91.
6. Lotan R. Immunomodulatory effects of retinoids. J Nutr Growth Cancer. 1986; 3:57–65.
8. Hofmann C, Eichele G. Retinoids in development. Sporn MB, Roberts AB, Goodman DS, editors. The retinoids: biology, chemistry and medicine. 2nd ed.New York, NY: Raven Press;1994. 387-442.
9. Israel H, Odziemiec C, Ballow M. The effects of retinoic acid on immunoglobulin synthesis by human cord blood mononuclear cells. Clin Immunol Immunopathol. 1991; 59:417–25.

10. Wang W, Ballow M. The effects of retinoic acid on in vitro immunoglobulin synthesis by cord blood and adult peripheral blood mononuclear cells. Cell Immunol. 1993; 148:291–300.
11. Ballow M, Wang W, Xiang S. Modulation of B-cell immunoglobulin synthesis by retinoic acid. Clin Immunol Immunopathol. 1996; 80:S73–81.

12. Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994; 152:1515–22.
13. Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, et al. Retinoid treatment of experimental allergic encephalomyelitis IL-4 production correlates with improved disease course. J Immunol. 1995; 154:450–8.
14. Stephensen C, Rasooly R, Jiang X, Ceddia M, Weaver C, Chandraratna R, et al. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol. 2002; 168:4495–503.
15. Lee HY, Dohi DF, Kim YH, Walsh GL, Consoli U, Andreeff M, et al. All-trans retinoic acid converts E2F into a transcriptional suppressor and inhibits the growth of normal human bronchial epithelial cells through a retinoic acid receptor-dependent signaling pathway. J Clin Invest. 1998; 101:1012–9.
16. Sun SY, Yue P, Lotan R. Implication of multiple mechanisms in apoptosis induced by the synthetic retinoid CD437 in human prostate carcinoma cells. Oncogene. 2000; 19:4513–22.

17. Wan YJ, Cai Y, Magee TR. Retinoic acid differentially regulates retinoic acid receptor-mediated pathways in the Hep3B cell line. Exp Cell Res. 1998; 238:241–7.

18. Agadir A, Shealy YF, Hill DL, Zhang X. Retinyl methyl ether down-regulates activator protein 1 transcriptional activation in breast cancer cells. Cancer Res. 1997; 57:3444–50.
19. Liu PT, Krutzik SR, Kim J, Modlin RL. All-trans Retinoic acid down-regulates TLR2 expression and function. J Immunol. 2005; 174:2467–70.
20. Gudas LJ, Sporn MB, Roberts AB. Cellular biology and biochemistry of the retinoids. Sporn MB, Roberts AB, Goodman DS, editors. The retinoids: biology, chemistry and medicine. 2nd ed.New York, NY: Raven Press;1994. p. 443–50.
21. Ross AC, Stephensen CB. Vitamin A and retinoids in antiviral responses. FASEB J. 1996; 10:979–85.

22. Ross AC, Ha¨mmerling UG. Retinoids and the immune system. Sporn MB, Roberts AB, Goodman DS, editors. The retinoids: biology, chemistry and medicine. 2nd ed.New York, NY: Raven Press;1994. p. 521–43.
23. Ballow M, Xiang S, Wang W, Brodsky L. The effects of retinoic acid on immunoglobulin synthesis: role of interleukin 6. J Clin Immunol. 1996; 16:171–9.

24. Ballow M, Wang W. Retinoic acid (RA) induced enhancing effects on immunoglobulin (Ig) synthesis of human B-cells. Pediatr Res. 1993; 33:151A.
25. Ballow M, Xiang S, Greenberg SJ, Brodsky L, Allen C, Rich G. Retinoic acid-induced modulation of IL-2mRNA production and IL-2 receptor expression on T Cells. Int Arch Allergy Immunol. 1997; 113:167–9.
26. Matikainen S, Serkkola E, Hurme M. Retinoic acid enhances IL-1βexpression in myeloid leukemia cells and in human monocytes. J Immunol. 1991; 147:162–7.
Go to : 
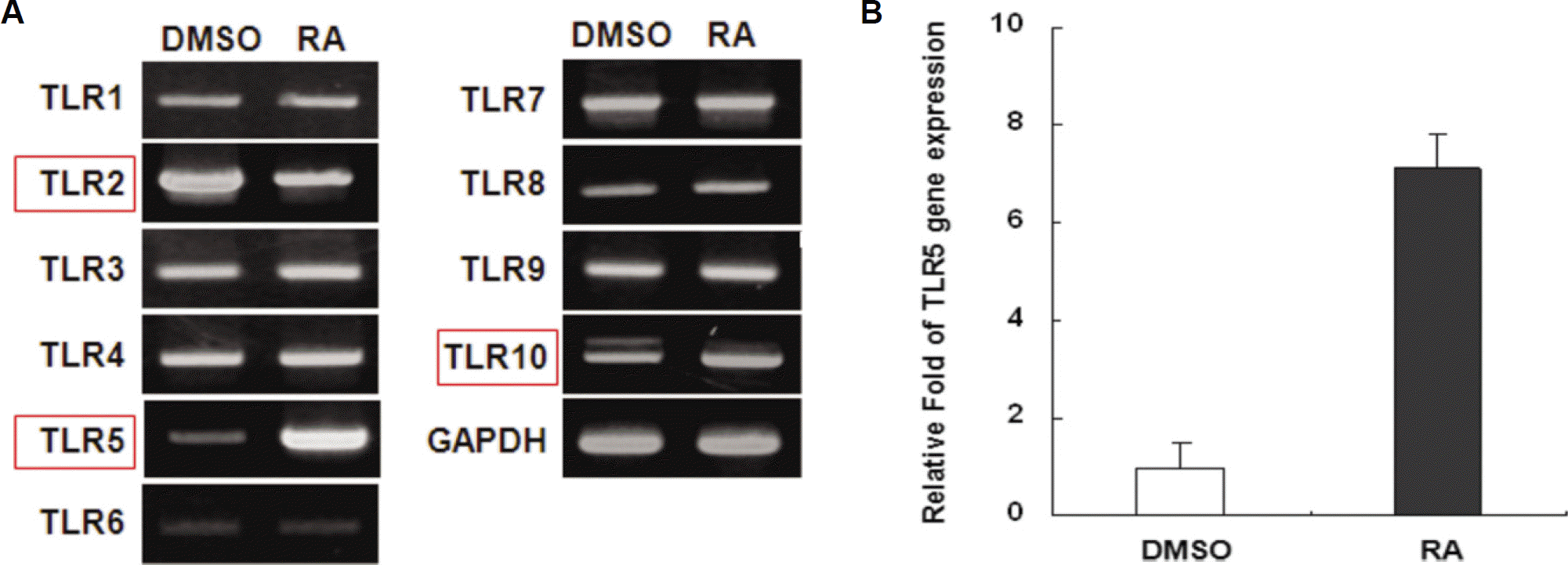 | Fig. 1.Effect of all-trans retinoic acid on the expression of Toll-like receptors in THP-1 cells. A. THP-1 cells were stimulated with a 10−8 M concentration of RA for 24 hours. Total RNAs were isolated from the cells and analyzed for human TLR mRNA expression by RT-PCR. B. Gene expression of human TLR-5 was analyzed at the same condition by real-time PCR. (DMSO: dimethyl sulfoxide only, RA: retinoic acid, TLR: Toll-like receptor, GAPDH: glyceraldehyde 3 phosphate dehydrogenase, THP: human monocytic leukemia cell line, RT-PCR: reverse transcription polymerase chain reaction) |
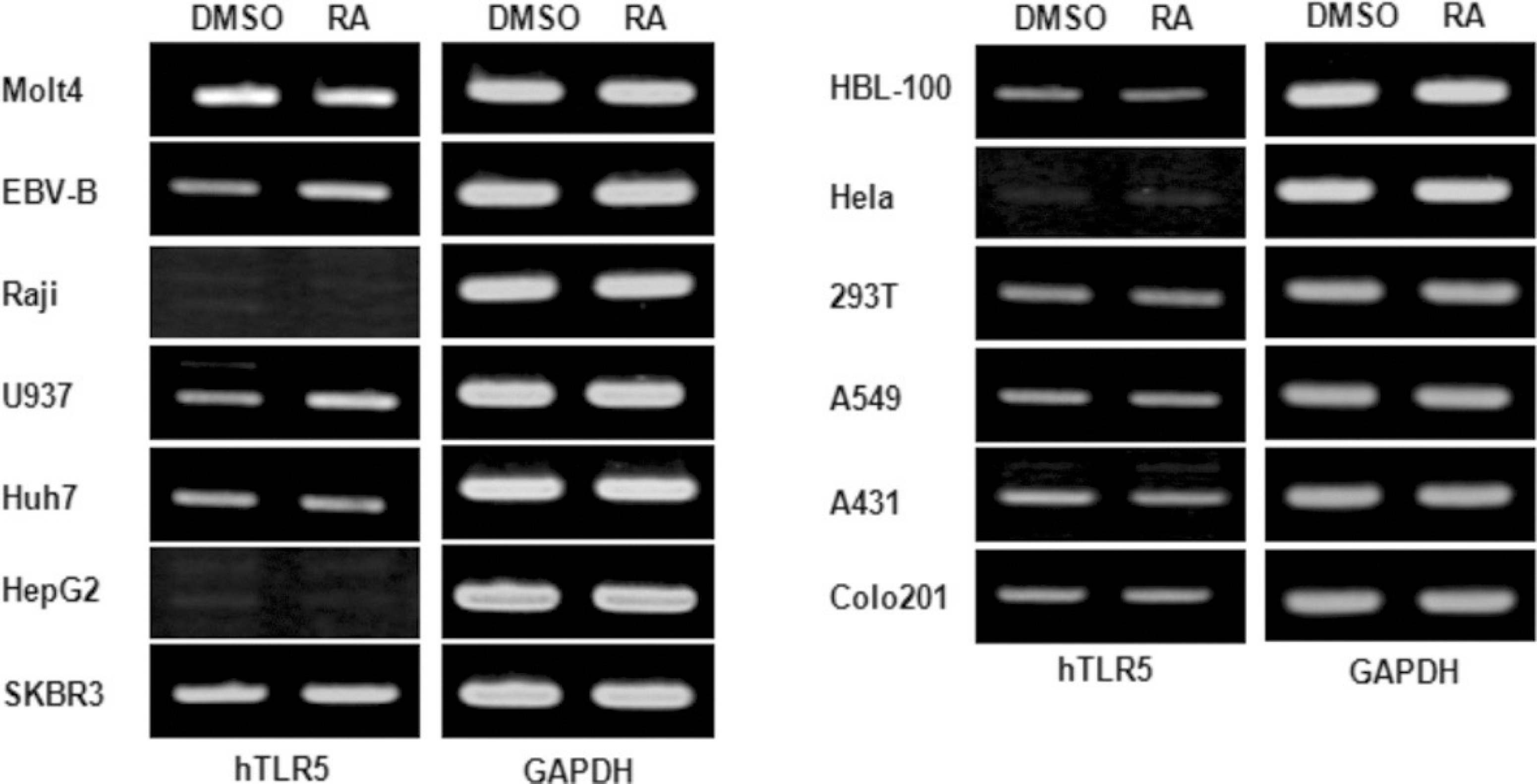 | Fig. 2.Effects of all-trans retinoic acid on the expression of human TLR-5 gene on the various human cell lines. The various human immune and cancer cell lines including Molt4, EBV-B, Raji, U937, Huh7, HepG2, SKBR3, HBL-100, Hela, 293T, A549, A431, Colo201 cell were stimulated with a 10−8 M concentration of atRA for 24 hours. Total RNAs were isolated from the cells and analyzed for human TLR-5 mRNA expression by RT-PCR. (DMSO: dimethyl sulfoxide only, RA: retinoic acid, GAPDH: glyceraldehyde 3 phosphate dehydrogenase, TLR: Toll-like receptor, RT-PCR: reverse transcription polymerase chain reaction) |
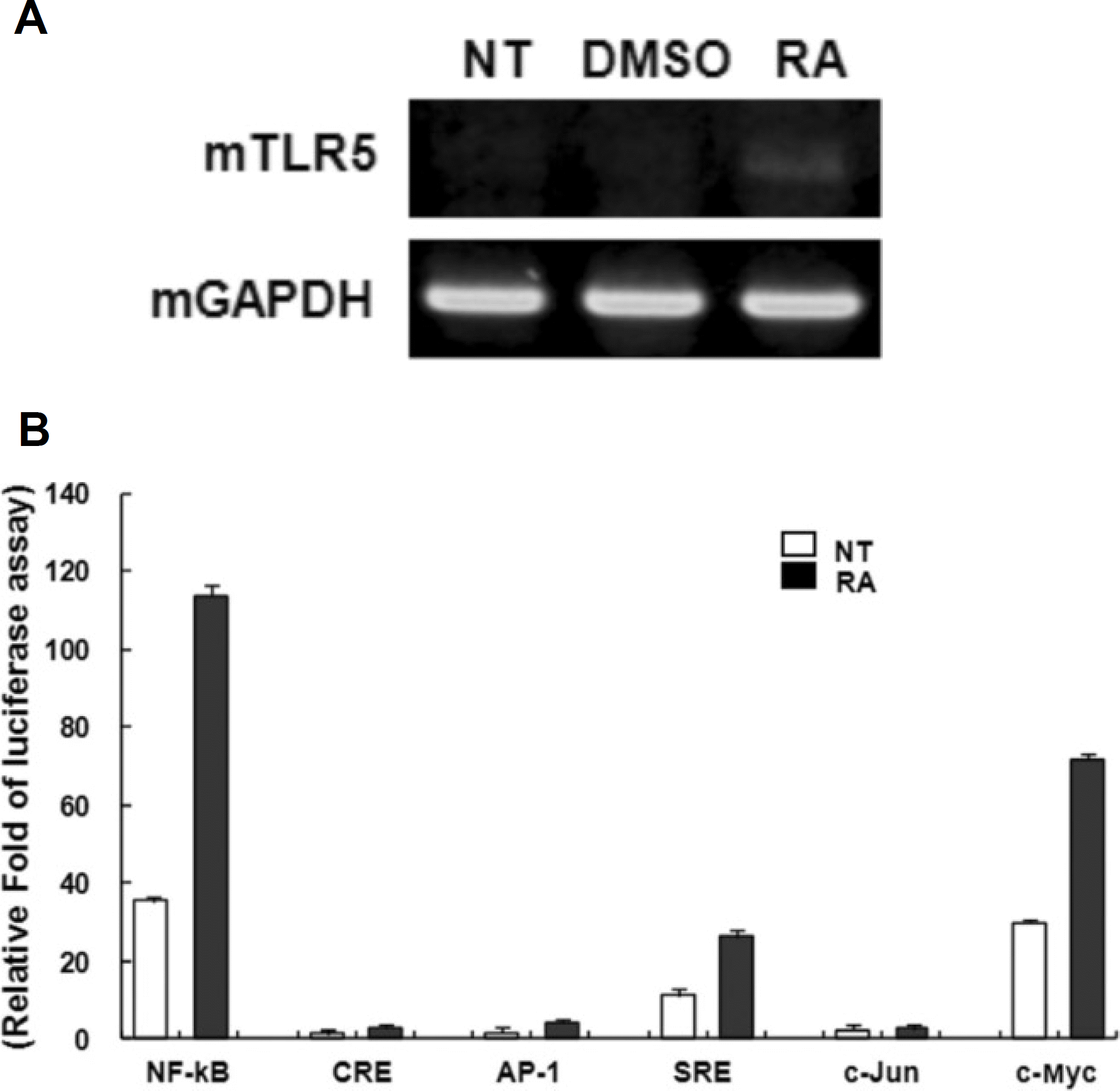 | Fig. 3.All-trans retinoic acid (atRA) induces the expression of mouse TLR-5 gene on RAW264.7 cells. A. The mouse RAW264.7 cells were stimulated with a 10−8 M concentration of atRA for 24 hours. Total RNAs were isolated from the cells and analyzed for mouse TLR-5 mRNA expression by RT-PCR. B. Raw264.7 cells were transiently co-transfected with various promoter constructs luciferase vector such as NF-kB, CRE, AP-1, SRE, c-Jun, and c-Myc with pRL CMV. After 6 hours of transfection, the cells were left untreated or treated with 10−8 M atRA for the last 24 hours. Relative luciferase activity was determined as described in materials and methods. Results are represented as means ±SD of a representative experiment performed in triplicate. (DMSO: dimethyl sulfoxide only, RA: retinoic acid, GAPDH: glyceraldehyde 3 phosphate dehydrogenase, TLR: Toll-like receptor, RAW: mouse leukemia monocyte macrophage cell line, RT-PCR: reverse transcription polymerase chain reaction, CMV: cytomegalovirus, SD: standard deviation) |
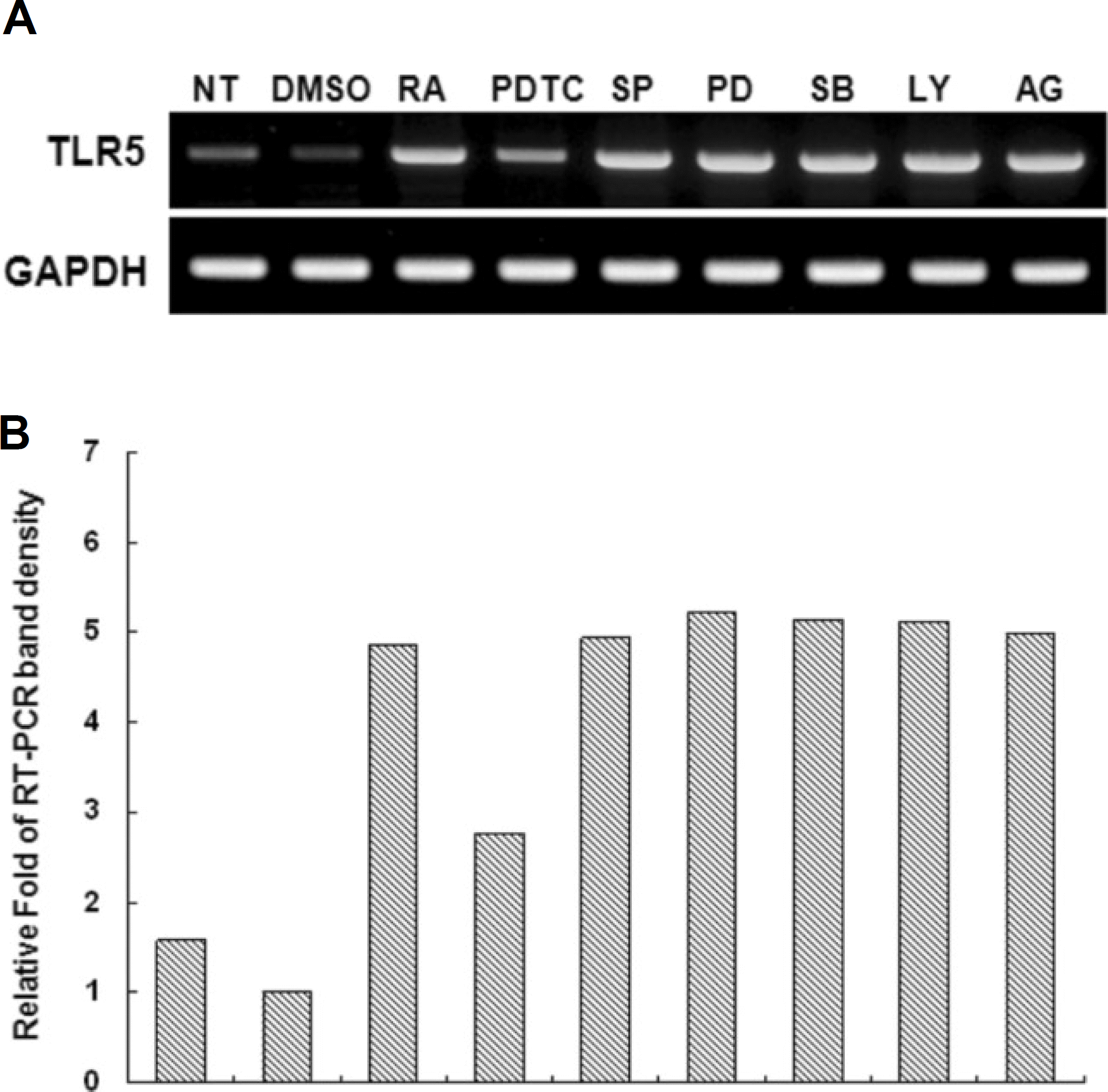 | Fig. 4.Effect of various signaling inhibitors on all-trans retinoic acid (atRA)-induced TLR-5 expression. A. THP-1 cells were pretreated for 0.5 hour with the indicated inhibitors and then stimulated with 10−8 M atRA in the presence or absence of the same inhibitor for an additional 24 hours. Total RNAs were prepared and analyzed by RT-PCR for TLR-5 or β-actin. (NT: no treatment, DMSO: dimethyl sulfoxide only, PDTC: pyrrolidine dithiocarbamate (NF-kB inhibitor), SP: SP600125 (JNK inhibitor), PD: PD98059 (extracellular signal-regulated kinase inhibitor), SB: SB203580 (p38 inhibitor), LY: LY294002 (PI3K inhibitor), AG: AG490 (JAKII inhibitor), GAPDH: glyceraldehyde 3 phosphate dehydrogenase, TLR: Toll-like receptor, THP: human monocytic leukemia cell line, RT-PCR: reverse transcription polymerase chain reaction) |
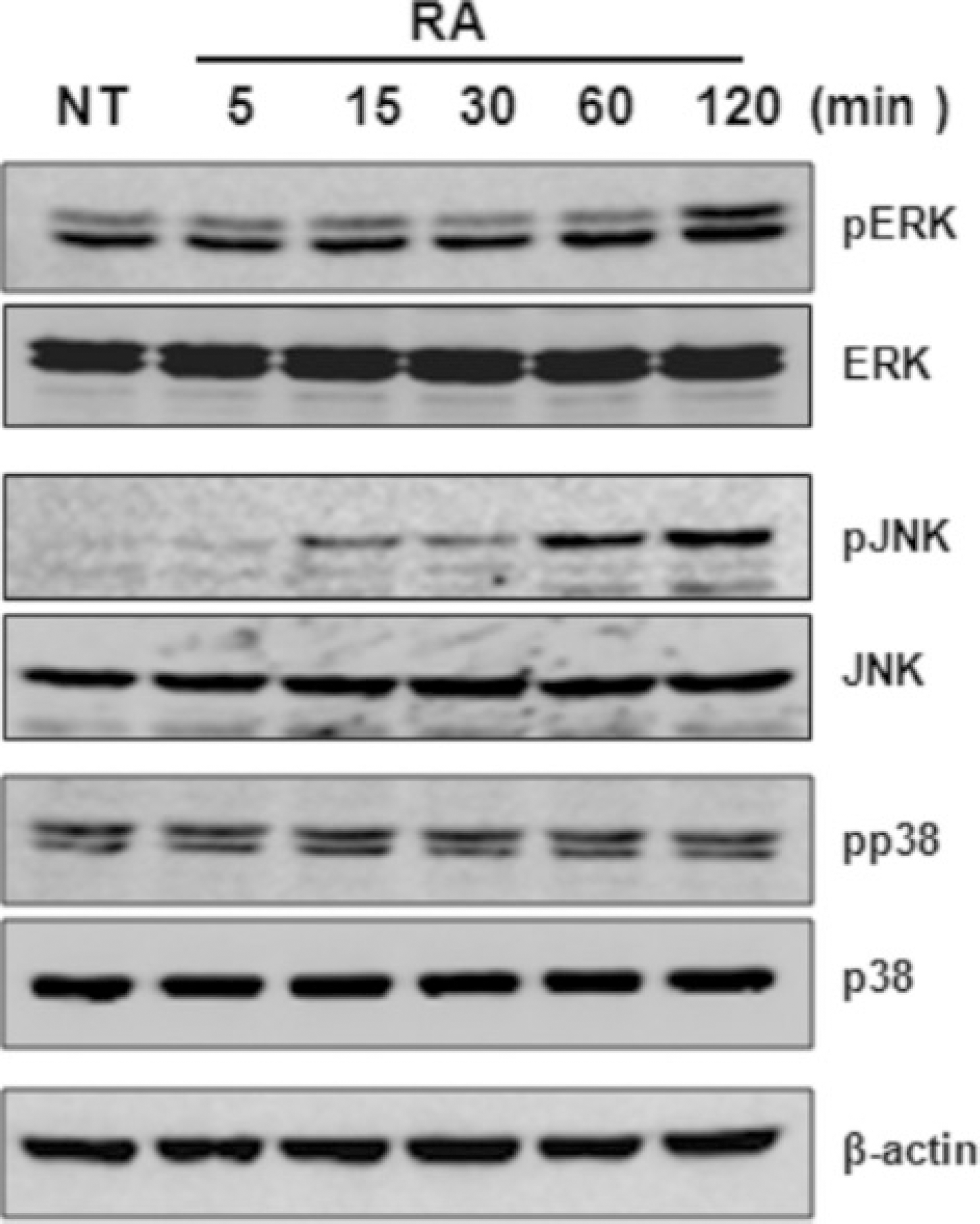 | Fig. 5.Effects of all-trans retinoic acid (atRA) on the activation of ERK, JNK, and p38 MAPK in THP-1 cells, THP-1 cells were serum-starved for 12 hours, stimulated with atRA (10−8 M) for the indicated times, and then lysed. Cell lysates were resolved by SDS-PAGE and then subjected to Western blotting withan antibody against phosphorylated ERK, JNK, and p38 MAPK. The same membrane was stripped and reprobed with anti-ERK, anti-JNK, anti-p38 MAPK, and anti-actin antibody. (THP: human monocytic leukemia cell line, NT: no treatment, RA: retinoic acid, ERK: extracellular signal-regulated kinase, JNK: MAPK: SDS-PAGE:) |
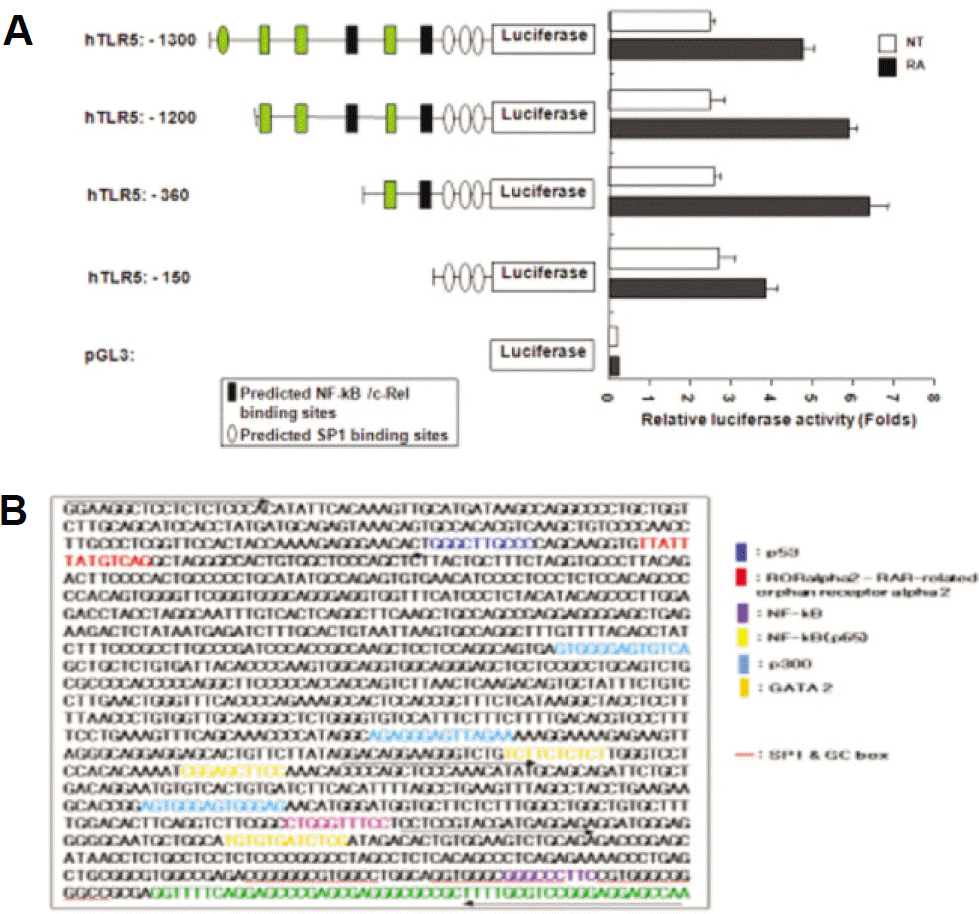 | Fig. 6.Promoter constructs and delineation of human TLR-5 promoter region. A. THP-1 cells were transiently cotrans-fected with various promoter constructs or empty luciferase vector with pRL CMV to compare transfection efficiencies. After transfection for 6 hours the cells were left untreated or treated with 10−8 M atRA for the last 24 hours. Relative luciferase activity was determined as described in materials and methods. Results are represented as means ±SD of a representative experiment performed in triplicate. B.Nucleotide sequence of the promoter region of human TLR-5 gene. The 1,300 bp sequence of the 5’-flanking region of TLR-5 is shown. The putative transcription start site is indicated by +1 as shown mRNA sequences from GenBank accession number NM_003268. The arrow indicates the transcription start site. Underlined sequences are possible transcription factor binding sites, as predicted by GenomeNet. (THP: human monocytic leukemia cell line, NT: no treatment, RA: retinoic acid, TLR: Toll-like receptor, CMV: cytomegalovirus, SD: standard deviation) |
Table 1.
Oligonucleotide primer sequence used in this study and products size of the amplified RT-PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download