Abstract
Introduction
This study examined the effect of recombinant human bone morphogenetic protein (rhBMP)-2 and β -tricalcium phosphate (β -TCP) on new bone formation in a rabbit calvarium using a rapid prototype titanium cap (RP Ti cap).
Materials and Methods
Eight New Zealand white rabbits were used in this study. Hemispherical RP Ti caps (10 mm in diameter) were implanted subperiosteally on the rabbit calvaria. β -TCP was filled in the RP Ti cap in the control group, and rhBMP-2 soaked β -TCP was used in experimental group. The rabbits were sacrificed 2 and 4 weeks after the operation. The volume and pattern of newly formed bone was analyzed by micro computed tomography (CT).
Results
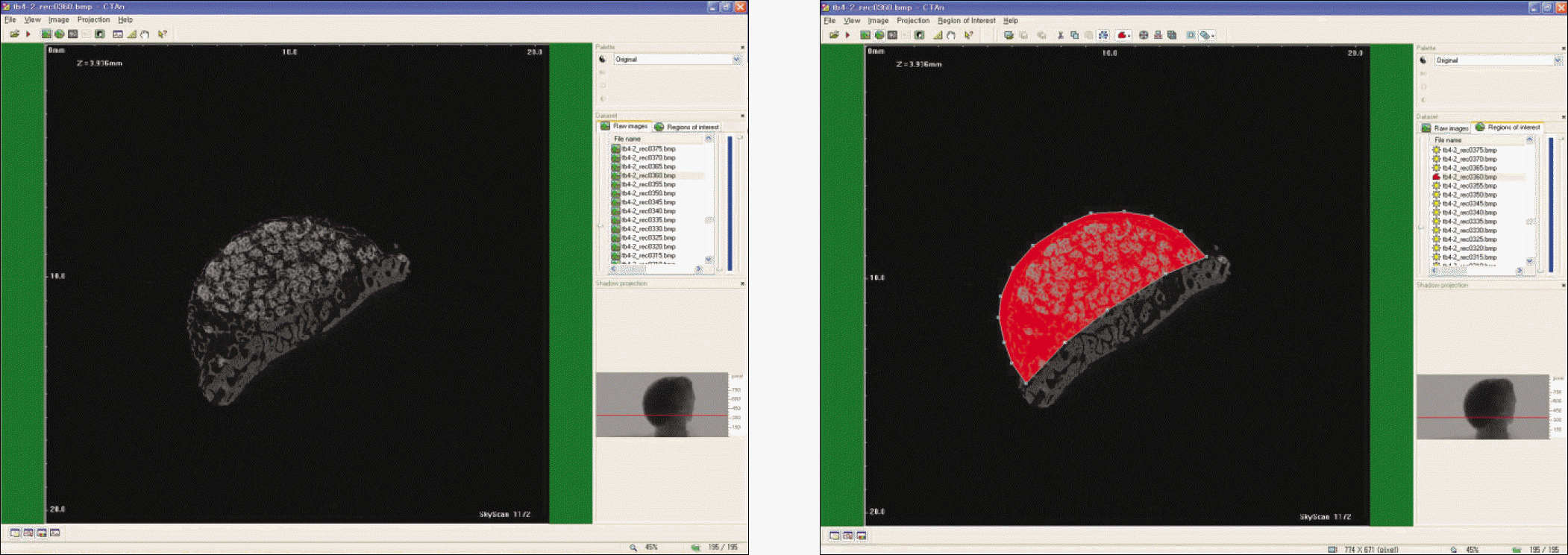
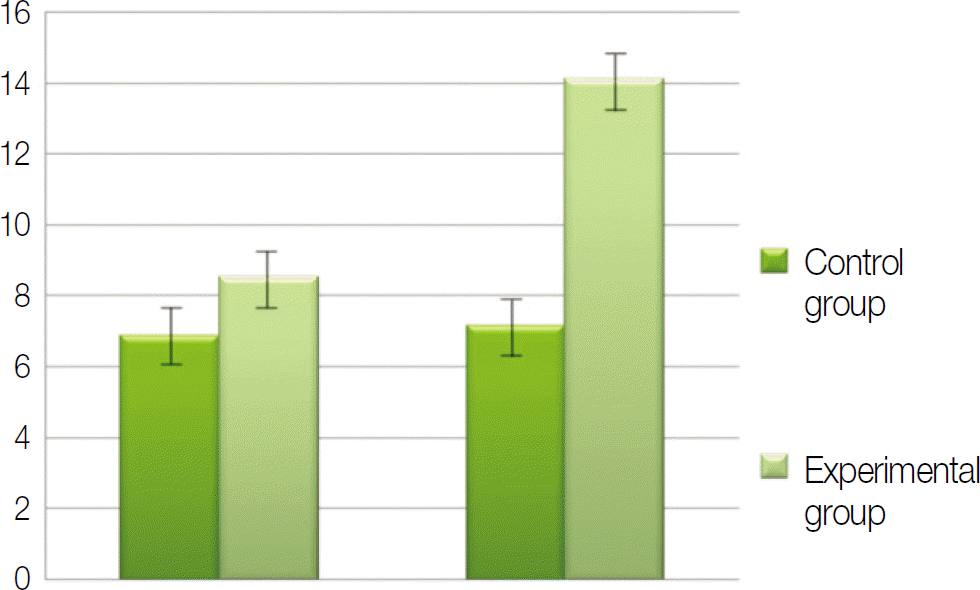
Macroscopically, there were no abnormal findings in any of the animals. The micro CT images revealed new bone from the calvaria that expanded gradually toward the top of the titanium cap, particularly along the inner surface of the titanium cap in the experimental group at 4 weeks after grafting. There was no significant difference in new bone volume ratio between the control and experimental groups at 2 weeks after grafting. There was a statistically significant difference in the new bone volume ratio between the experimental (14.1±1.8 %) and control (7.2±1.5 %) groups at 4 weeks after grafting (P<0.01).
Go to : 
REFERENCES
1. Dahlin C. Scientific background of guided bone regeneration. Buser D, Dahlin C, Schenk RK, editors. Guided bone regeneration in implant dentistry. Chicago: Quintessence Publishing Co. Inc.;1994. p. 31–48.
2. Kostopoulos L, Karring T. Augmentation of the rat mandible using guided tissue regeneration. Clin Oral Implants Res. 1994; 5:75–82.

3. Lundgren D, Lundgren AK, Sennerby L, Nyman S. Augmentation of intramembraneous bone beyond the skeletal envelope using an occlusive titanium barrier. An experimental study in the rabbit. Clin Oral Implants Res. 1995; 6:67–72.

4. Yamada Y, Nanba K, Ito K. Effects of occlusiveness of a titanium cap on bone generation beyond the skeletal envelope in the rabbit calvarium. Clin Oral Implants Res. 2003; 14:455–63.

5. van Steenberghe D, Johansson C, Quirynen M, Molly L, Albrektsson T, et al. Bone augmentation by means of a stiff occlusive titanium barrier. Clin Oral Implants Res. 2003; 14:63–71.

6. Nishida T, Yamada Y, Murai M, Shimizu Y, Oshikawa M, Ito K. Effects of bioactive glass on bone augmentation within a titanium cap in rabbit parietal bone. J Periodontol. 2006; 77:983–9.

7. Min S, Sato S, Murai M, Okuno K, Fujisaki Y, Yamada Y, et al. Effects of marrow penetration on bone augmentation within a titanium cap in rabbit calvarium. J Periodontol. 2007; 78:1978–84.

8. Tamura T, Fukase Y, Goke E, Yamada Y, Sato S, Nishiyama M, et al. Three-dimensional evaluation for augmented bone using guided bone regeneration. J Periodontal Res. 2005; 40:269–76.

9. Yamada Y, Tamura T, Hariu K, Asano Y, Sato S, Ito K. Angiogenesis in newly augmented bone observed in rabbit calvarium using a titanium cap. Clin Oral Implants Res. 2008; 19:1003–9.

10. Min S, Sato S, Saito M, Ebihara H, Arai Y, Ito K. Micro-computerized tomography analysis: dynamics of bone augmentation within a titanium cap in rabbit calvarium. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:892–5.

11. Molly L, Quirynen M, Michiels K, van Steenberghe D. Comparison between jaw bone augmentation by means of a stiff occlusive titanium membrane or an autologous hip graft: a retrospective clinical assessment. Clin Oral Implants Res. 2006; 17:481–7.

12. Schmid J, Wallkamm B, Ha ¨mmerle CH, Gogolewski S, Lang NP. The significance of angiogenesis in guided bone regeneration. A case report of a rabbit experiment. Clin Oral Implants Res. 1997; 8:244–8.

13. Tamura K, Sato S, Kishida M, Asano S, Murai M, Ito K. The use of porous beta-tricalcium phosphate blocks with platelet-rich plasma as an onlay bone graft biomaterial. J Periodontol. 2007; 78:315–21.
14. Ogose A, Hotta T, Hatano H, Kawashima H, Tokunaga K, Endo N, et al. Histological examination of beta-tricalcium phosphate graft in human femur. J Biomed Mater Res. 2002; 63:601–4.
16. Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996; 222:38–47.

17. Matsushita N, Terai H, Okada T, Nozaki K, Inoue H, Miyamoto S, et al. A new bone-inducing biodegradable porous beta-tricalcium phosphate. J Biomed Mater Res A. 2004; 70:450–8.
18. Alam I, Asahina I, Ohmamiuda K, Enomoto S. Comparative study of biphasic calcium phosphate ceramics impregnated with rhBMP-2 as bone substitutes. J Biomed Mater Res. 2001; 54:129–38.

19. Jingushi S, Urabe K, Okazaki K, Hirata G, Sakai A, Ikenoue T, et al. Intramuscular bone induction by human recombinant bone morphogenetic protein-2 with beta-tricalcium phosphate as a carrier: in vivo bone banking for muscle-pedicle autograft. J Orthop Sci. 2002; 7:490–4.
20. Laffargue P, Hildebrand HF, Rtaimate M, Frayssinet P, Amoureux JP, Marchandise X. Evaluation of human recombinant bone morphogenetic protein-2-loaded tricalcium phosphate implants in rabbits’ bone defects. Bone. 1999; 25(2 Suppl):55S–58S.

21. Urist MR, Nilsson O, Rasmussen J, Hirota W, Lovell T, Schmalzreid T, et al. Bone regeneration under the influence of a bone morphogenetic protein (BMP) beta tricalcium phosphate (TCP) composite in skull trephine defects in dogs. Clin Orthop Relat Res. 1987; (214):295–304.

22. Mu ¨ller R, van Campenhout H, van Damme B, van Der Perre G, Dequeker J, Hildebrand T, et al. Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and micro-computed tomography. Bone. 1998; 23:59–66.

Go to : 
 | Fig. 1.Rapid prototype titanium cap is 5 mm in height, 10 mm in diameter and 1 mm in thickness with rough surface. |
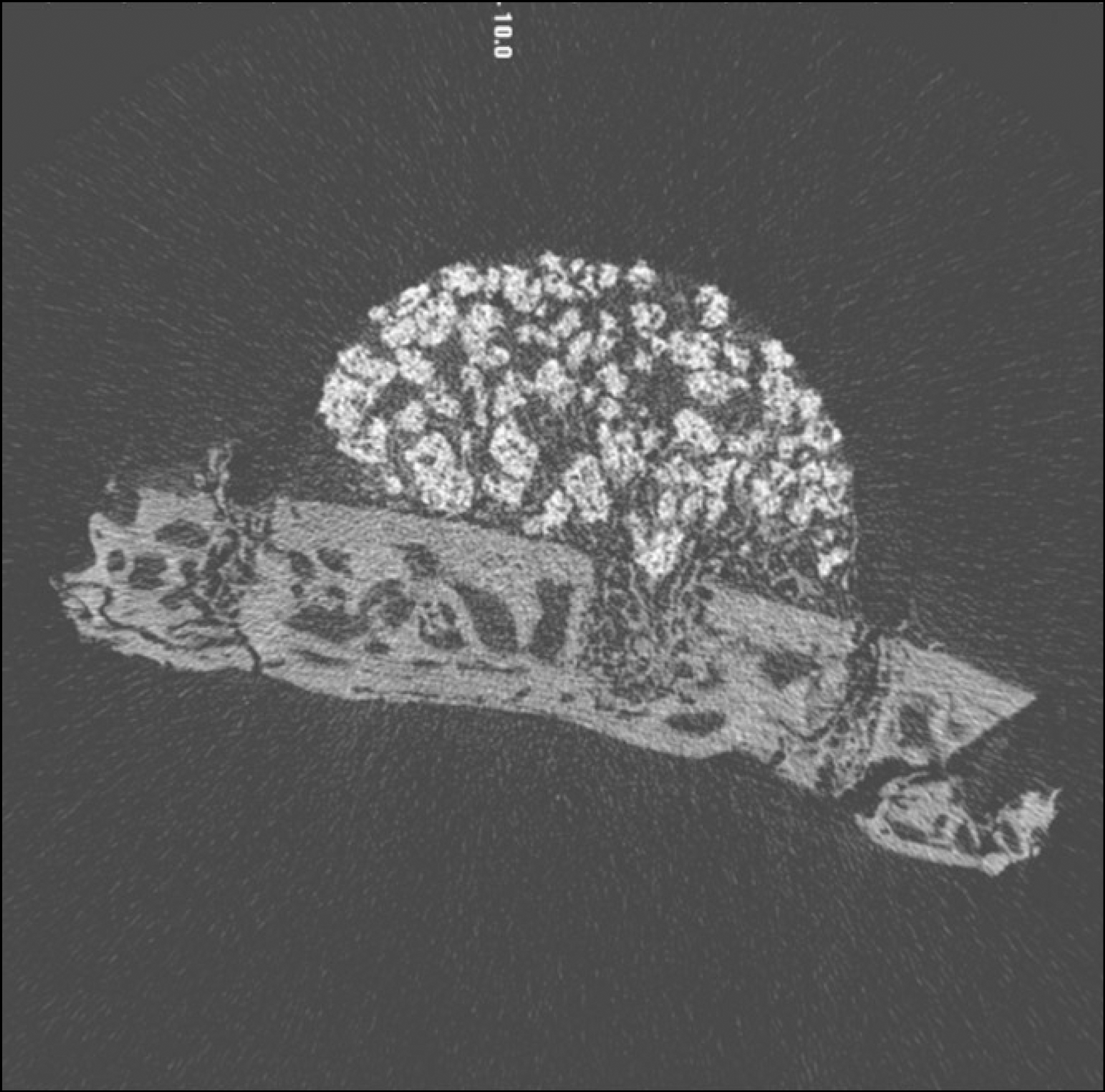 | Fig. 3.Micro CT images of 2 weeks after grafting in control group. (CT: computed tomography) |
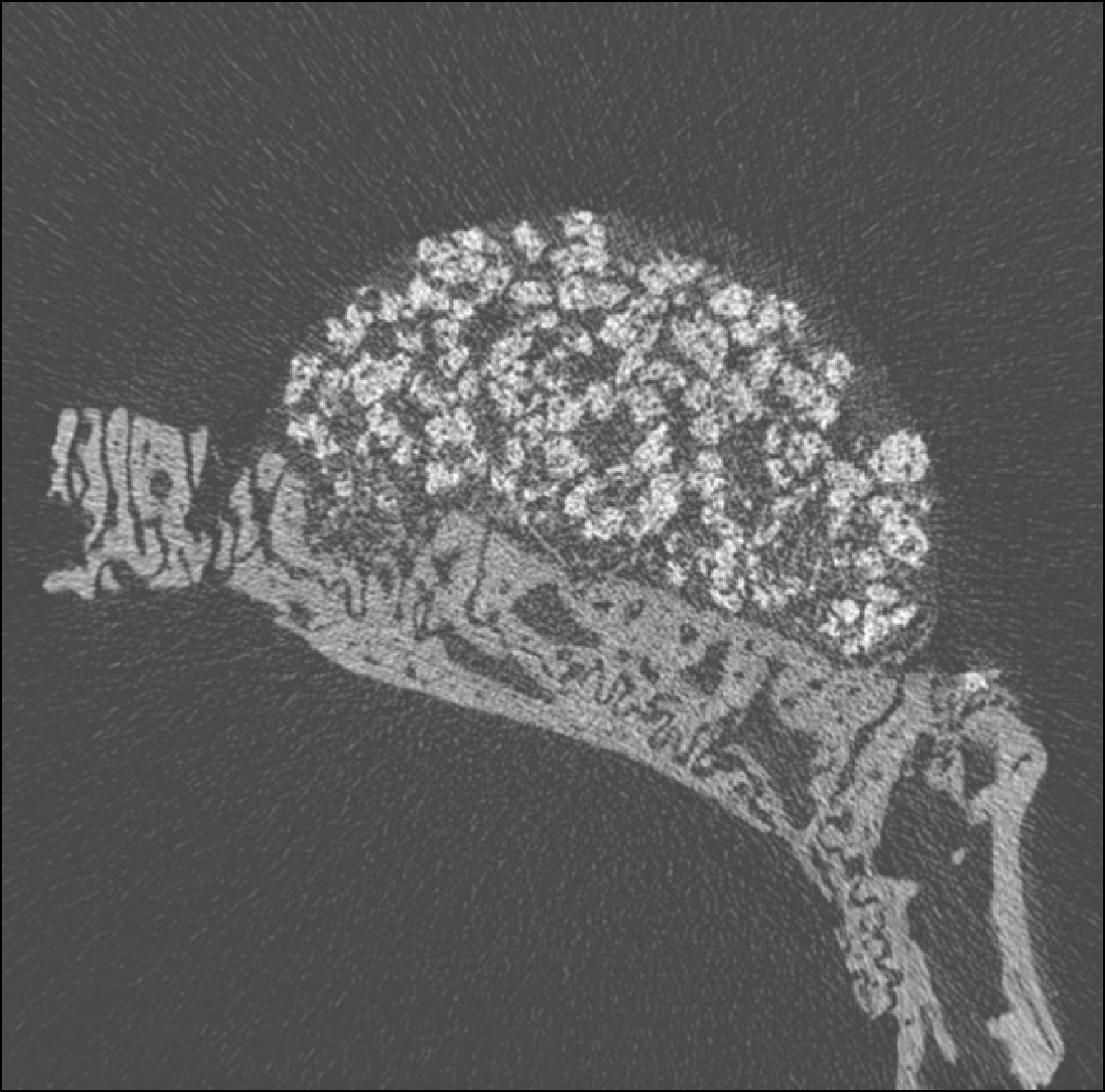 | Fig. 4.Micro CT images of 2 weeks after grafting in experimental group. (CT: computed tomography) |
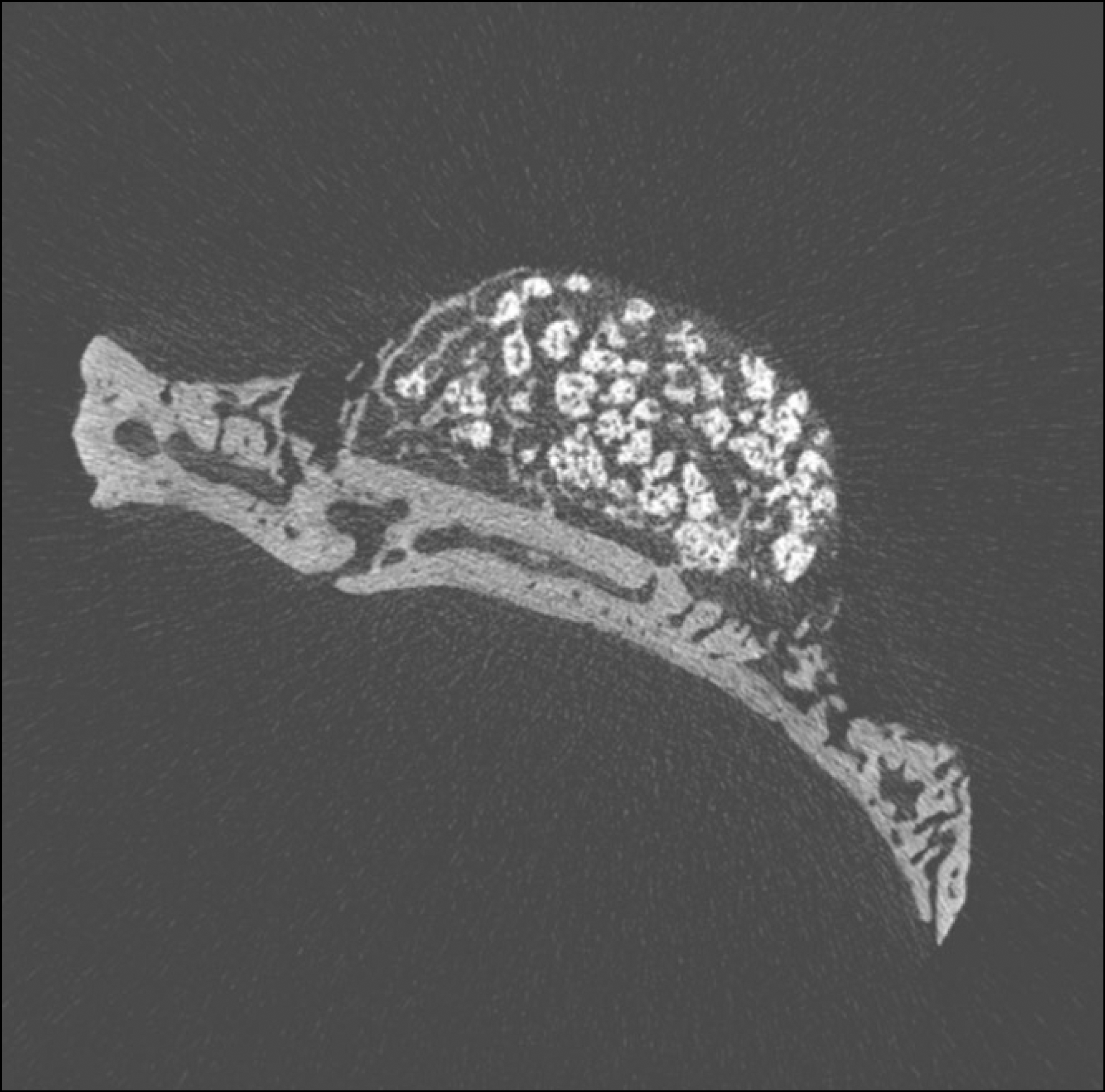 | Fig. 5.Micro CT images of 4 weeks after grafting in control group. (CT: computed tomography) |
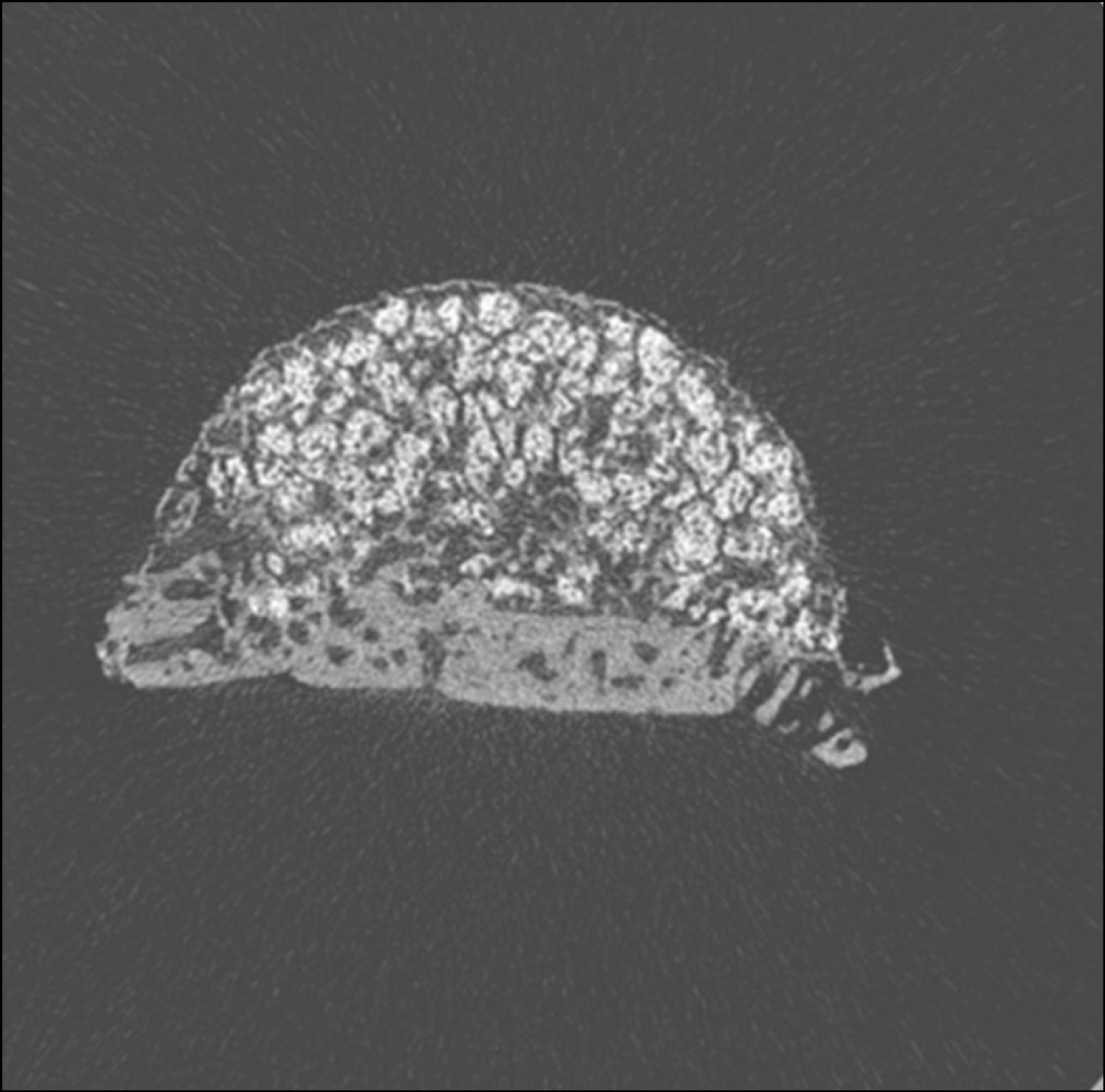 | Fig. 6.Micro CT images of 4 weeks after grafting in experimental group. (CT: computed tomography) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download