1. Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon, France: International Agency for Research on Cancer Press. 2005.
2. Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet. 2001; 10:687–92.

3. French SW, Dawson DW, Miner MD, Doerr JR, Malone CS, Wall R, et al. DNA methylation profiling: a new tool for evaluating hematologic malignancies. Clin Immunol. 2002; 103:217–30.

4. Ho A, Dowdy SF. Regulation of G(1) cell-cycle progression by oncogenes and tumor suppressor genes. Curr Opin Genet Dev. 2002; 12:47–52.

5. Rush LJ, Plass C. Alterations of DNA methylation in hematologic malignancies. Cancer Lett. 2002; 185:1–12.

6. Kresty LA, Mallery SR, Knobloch TJ, Song H, Lloyd M, Casto BC, et al. Alterations of p16(INK4a) and p14(ARF) in patients with severe oral epithelial dysplasia. Cancer Res. 2002; 62:5295–300.
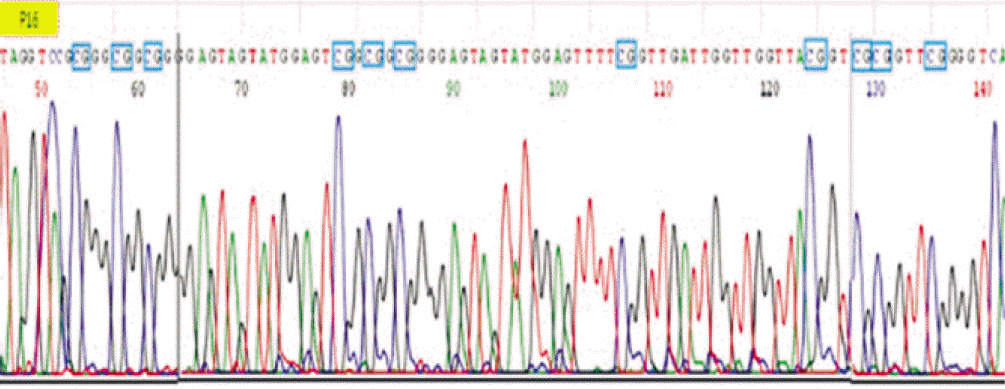
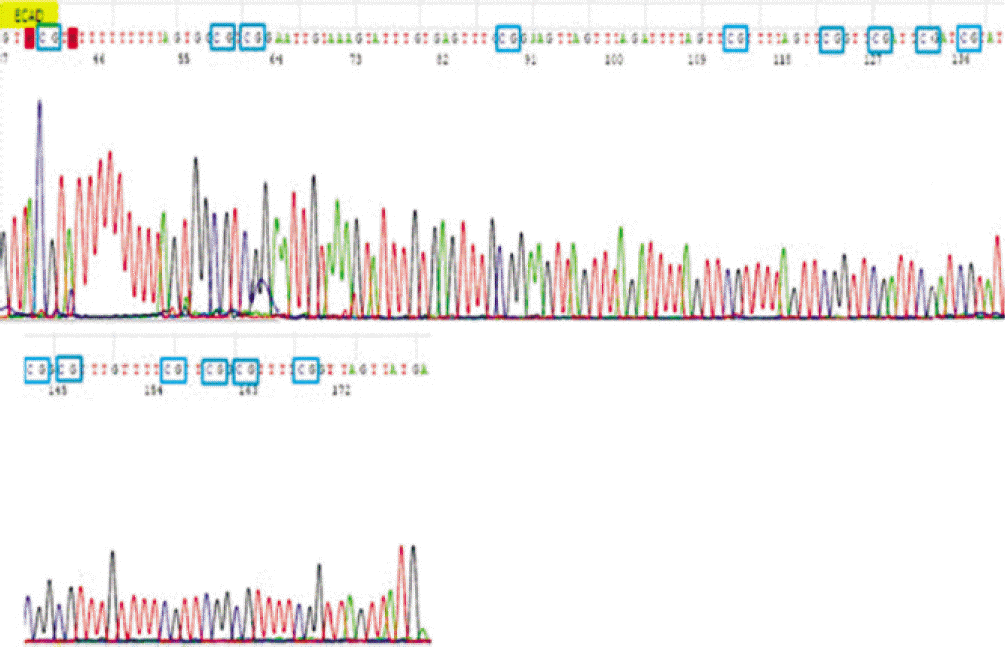
7. Viswanathan M, Tsuchida N, Shanmugam G. Promoter hypermethylation profile of tumor-associated genes p16, p15, hMLH1, MGMT and E-cadherin in oral squamous cell carcinoma. Int J Cancer. 2003; 105:41–6.

8. Abiko Y, Nagayasu H, Takeshima M, Yamazaki M, Nishimura M, Kusano K, et al. Ameloblastic carcinoma ex-ameloblastoma: report of a case-possible involvement of CpG island hypermethylation of the p16 gene in malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103:72–6.
9. Chen Q, Lipkina G, Song Q, Kramer RH. Promoter methylation regulates cadherin switching in squamous cell carcinoma. Biochem Biophys Res Commun. 2004; 315:850–6.

10. Pho SW, Kim YS, Park JY, Kim CH, Lee W, Park MK. The hypermethylation of E-cadherin gene in oral squamous cell carcinoma. J Korean Assoc Oral Maxillofac Surg. 2008; 34:135–40.
11. Auerkari EI. Methylation of tumor suppressor genes p16(INK4a), p7(Kip1) and E-cadherin in carcinogenesis. Oral Oncol. 2006; 42:5–13.
12. Sastre J, Muñoz M, Naval L, Adrados M. Ameloblastic carcinoma of the maxilla: report of a case. J Oral Maxillofac Surg. 2002; 60:102–4.

13. Verneuil A, Sapp P, Huang C, Abemayor E. Malignant ameloblastoma: classification, diagnostic and therapeutic challenges. Am J Otolaryngol. 2002; 23:44–8.

14. Thoma KH. Oral pathology: a histological, roentgenological, and clinical study of the diseases of the teeth, jaws, and mouth. 3rd ed.St. Louis: Mosby;1950.
15. Pindborg JJ, Kramer I, Torloni H. Histological typing of odontogenic tumors, jaw cysts, and allied lesions. Geneva, Switzerland: World Health Organization. 1972.
16. Slootweg PJ, Muller H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol. 1984; 57:168–76.

17. Zhao Y, Zhang S, Fu B, Xiao C. Abnormalities of tumor suppressor genes P16 and P15 in primary maxillofacial squamous cell carcinomas. Cancer Genet Cytogenet. 1999; 112:26–33.

18. Nodit L, Barnes L, Childers E, Finkelstein S, Swalsky P, Hunt J. Allelic loss of tumor suppressor genes in ameloblastic tumors. Mod Pathol. 2004; 17:1062–7.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download