Abstract
Introduction
Diabetes mellitus, as a major health problem for the elderly has been shown to alter the properties of the bone and impair bone healing around a titanium implant in both humans and animals. The aim of this study was to examine the effect of adipose-derived stem cells on the healing process around a titanium implant in streptozotocin-induced diabetic rats.
Materials and Methods
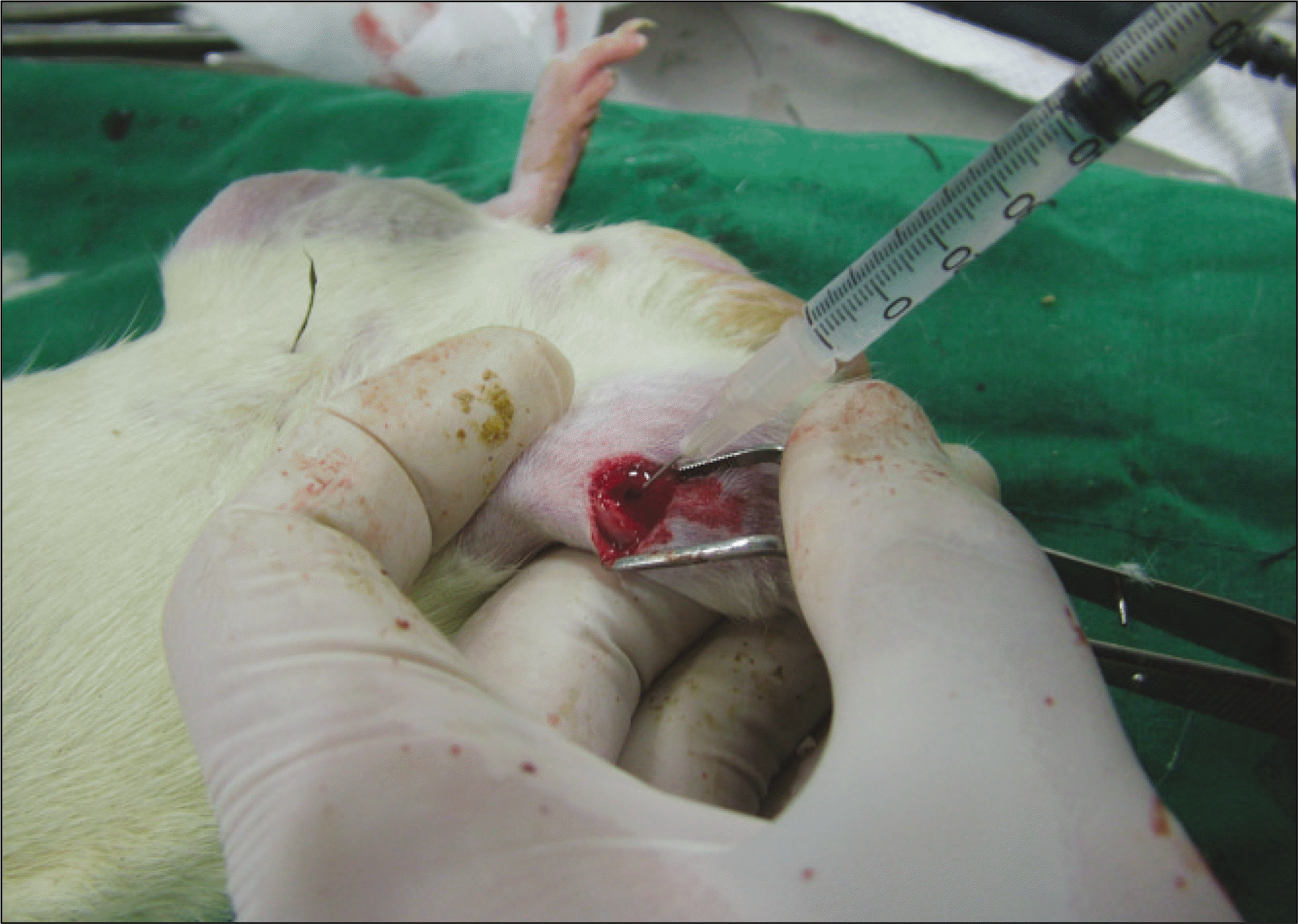
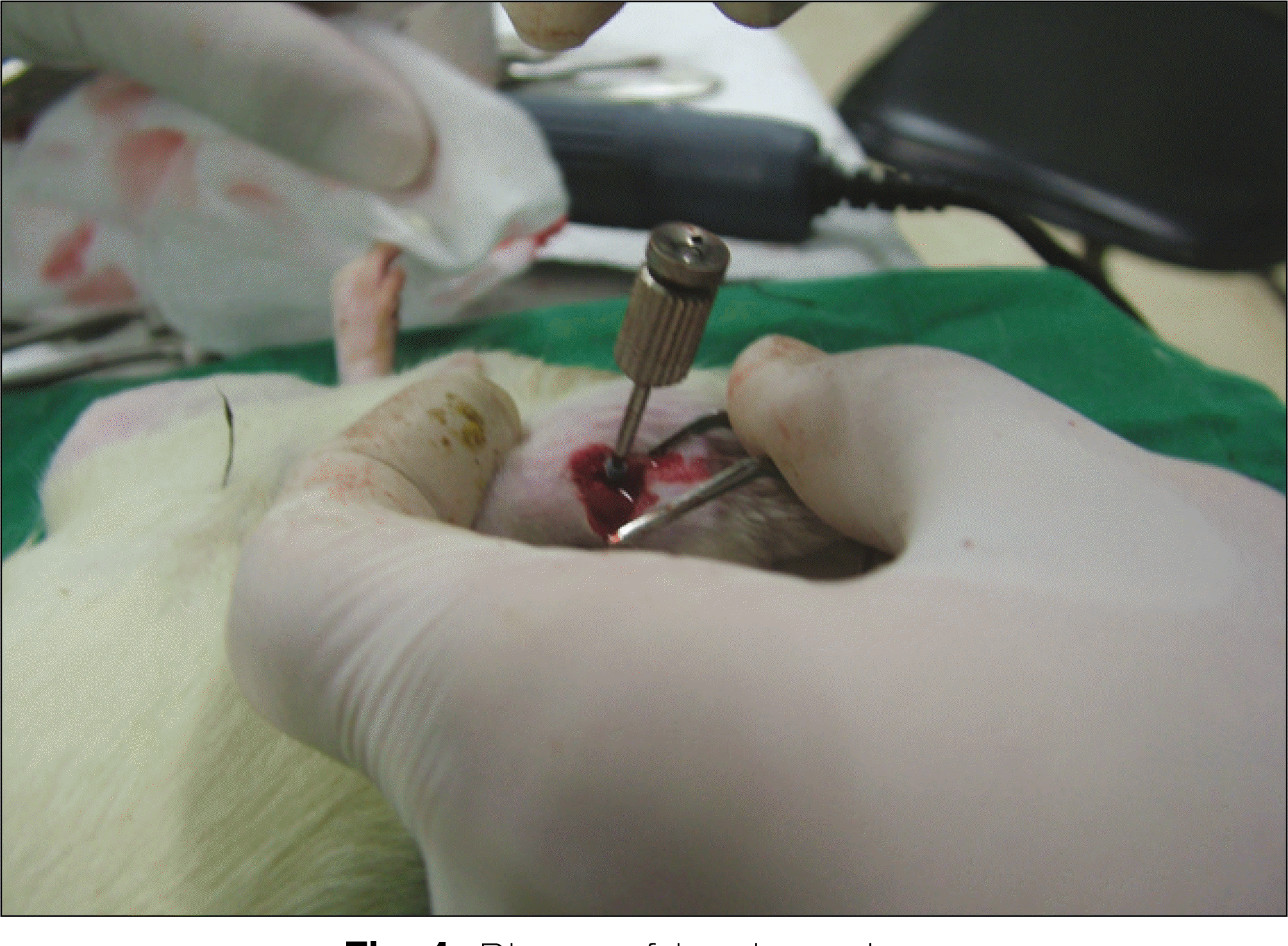
Thirteen rats were divided into two groups: adipose-derived stem cells injected group and a control group. A titanium screw implant (diameter: 2.0 mm, length: 3.5 mm) was placed into both tibia of 13 rats: 13 right tibia as the control group and 13 left tibia as the experimental group. The rats were sacrificed at different intervals (1, 2, and 4 weeks) after implantation for histopathology observations and immuno-histochemistric analysis.
Results
The histopathological findings revealed earlier new formed bone in the experimental group than the control group. In particular, at 1 week after implantation, the experimental group showed more newly formed bone and collagen around the implant than the control group. In immunohisto-chemistric analysis, osteoprotegerin (OPG) expression in the experimental group increased early compared to that of the control group until 2 weeks after implantation. However, after 2 weeks, OPG expression in the experimental group was similar to OPG expression in the control group. The receptor activator of nuclear factor κ B ligand (RANKL) expression in the experimental group increased early compared to that of the control group, and then decreased at 2 weeks. After 2 weeks, the level of RANKL expression was similar in both groups.
Go to : 
References
1. Zimmet P, Shaw J, Alberti KG. Preventing type 2 diabetes and the dysmetabolic syndrome in the real world: a realistic view. Diabet Med. 2003; 20:693–702.

2. Weiss RE, Gora A, Nimni ME. Abnormalities in the biosynthesis of cartilage and bone proteoglycans in experimental diabetes. Diabetes. 1981; 30:670–7.

3. Goodman WT, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes. 1984; 33:825–31.

4. Schwartz Z, Somers A, Mellonig JT, Carnes DL Jr, Dean DD, Cochran DL, et al. Abillity of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but gender. J Periodontol. 1998; 69:470–8.
5. Traianedes K, Russell JL, Edwards JT, Stubbs HA, Shanahan IR, Knaack D. Donor age and gender effects on osteoinductivity of demineralized bone matrix. J Biomed Mater Res B Appl Biomater. 2004; 70:21–9.

6. Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974; 17:331–40.
8. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrowderived mesenchymal progenitor cells. Exp Cell Res. 1998; 238:265–72.
9. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–7.

10. Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, et al. Combined effects of insulinlike growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003; 11:55–64.
11. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell based therapies. Tissue Eng. 2001; 7:211–28.
12. de Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multilineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003; 174:101–9.

13. Kwan Tat S, Padrines M, The ′ oleyre S, Heymann D, Fortun Y. Survey: IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004; 15:49–60.
14. Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. Survey: the molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004; 15:457–75.
15. Boyle WJ, Simonet WS, Lecey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–42.

16. McCracken M, Lemons JE, Rahemtulla F, Prince CW, Feldman D. Bone response to titanium alloy implants placed in diabetic rats. Int J Oral Maxillofac Implants. 2000; 15:345–54.
17. Nyomba BL, Verhaeghe J, Thomasset M, Lissens W, Bouillon R. Bone mineral homeostasis in spontaneously diabetic BB rats. Abnormal vitamin D metabolism and impaired active intestinal calcium absorption. Endocrinology. 1989; 124:565–72.
18. Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants. 1998; 13:620–9.

19. Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000; 5:157–65.

20. Mellado-Valero A, Ferrer Garcl′a JC, Herrera Ballester A, Labaig Rueda C. Effects of diabetes on the osseointegration of dental implants. Med Oral Patol Oral Cir Bucal. 2007; 12:E38–43.
21. Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent. 2000; 20:366–73.
22. Song L, Baksh D, Tuan RS. Mesenchymal stem cell-based cartilage tissue engineering: cells, scaffold and biology. Cytotherapy. 2004; 6:596–601.

23. Barry FP, Murphy JM. Mesenchymal stem cells: clinical application and biological characterization. Int J Biochem Cell Biol. 2004; 36:568–84.
24. Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004; 95:209–14.

25. Friedenstein AJ, Petrakova KV, Kurolesoval AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6:230–47.
26. Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999; 48:913–27.

27. Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001; 79:243–53.
Go to : 
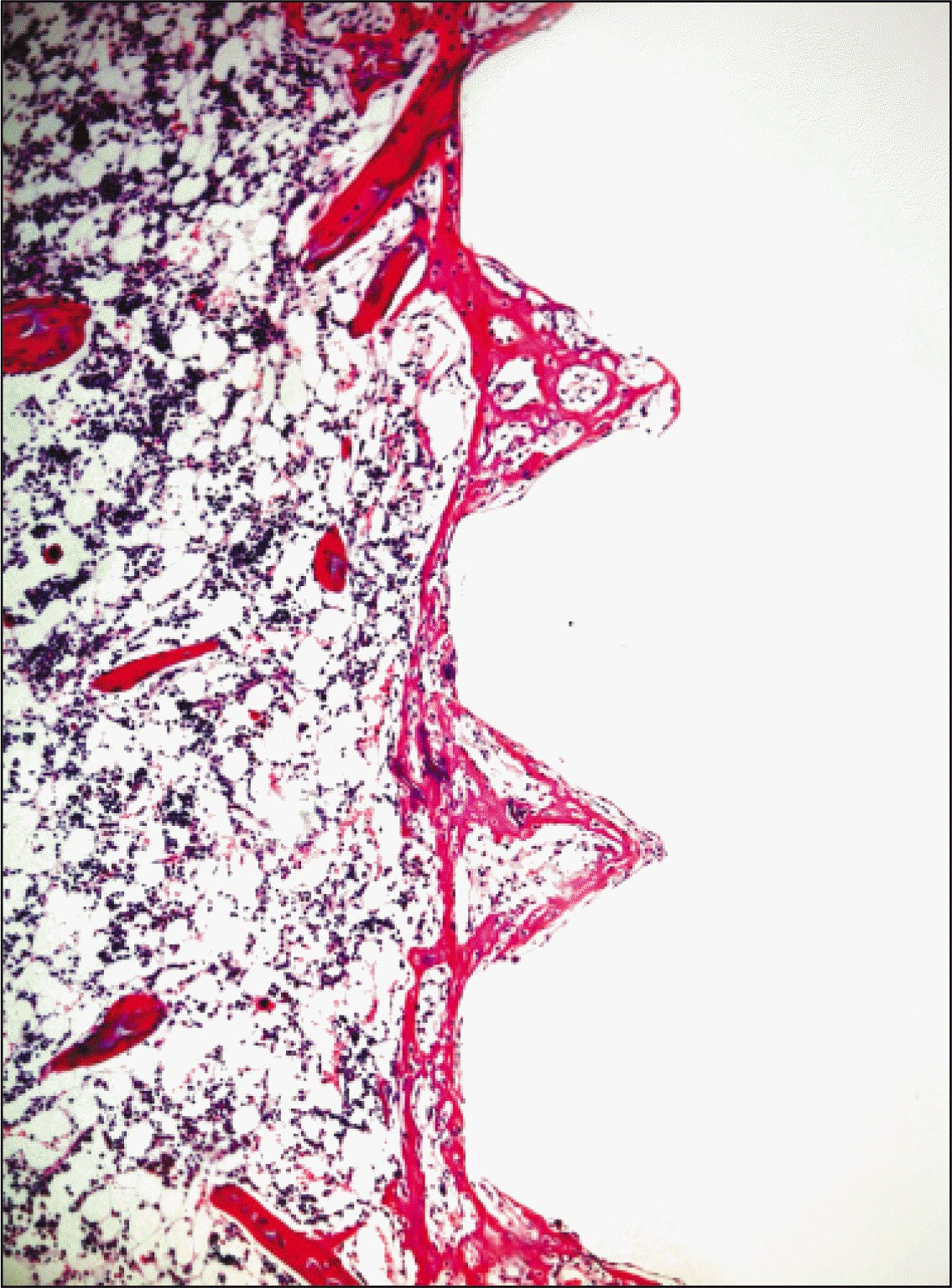 | Fig. 5.Microphotograph at 1 week after implantation in control rat.(H&E staining, original magnification ×100) |
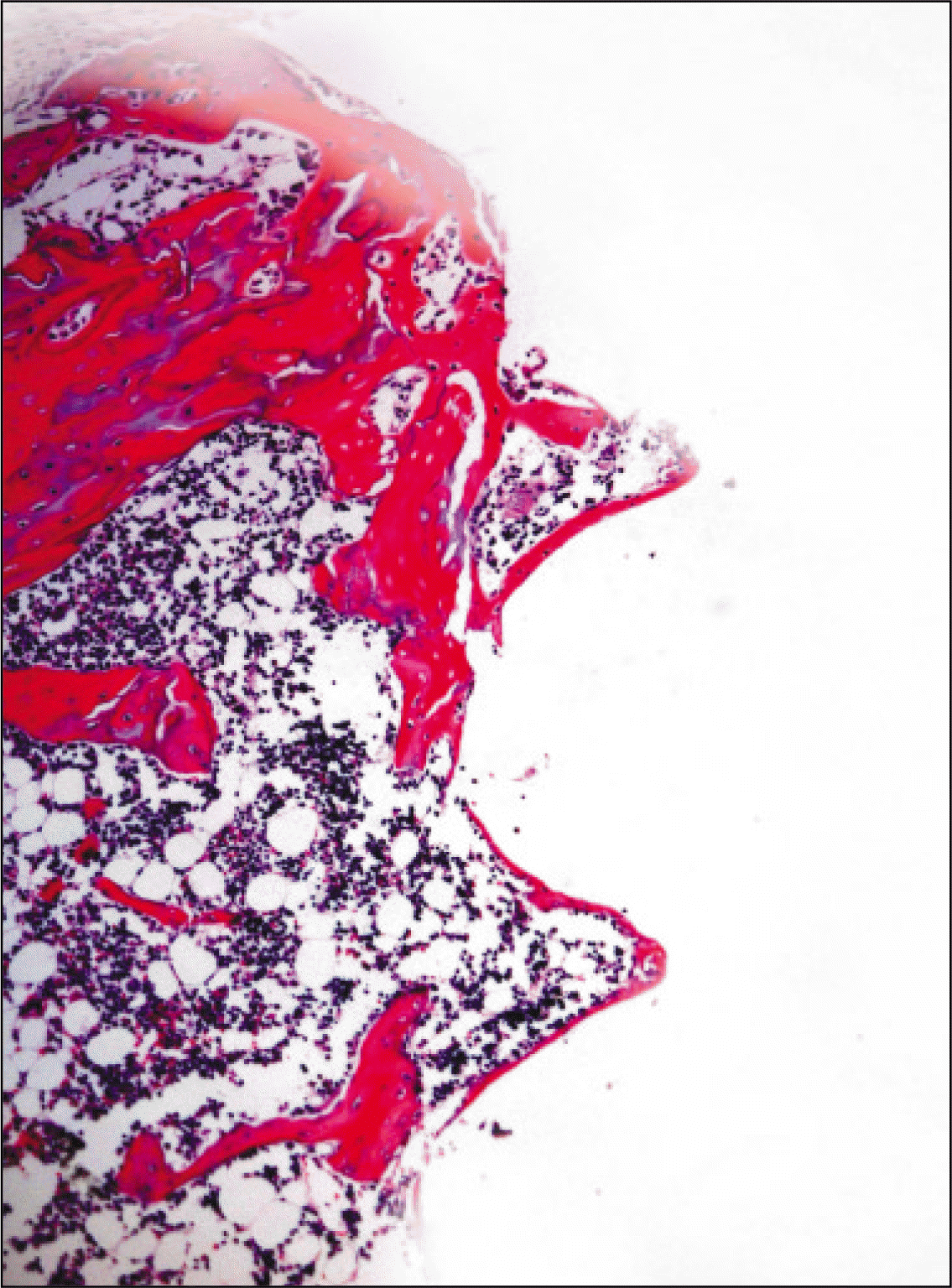 | Fig. 6.Microphotograph at 2 weeks after implantation in control rat.(H&E staining, original magnification ×100) |
 | Fig. 7.Microphotograph at 4 weeks after implantation in control rat.(H&E staining, original magnification ×100) |
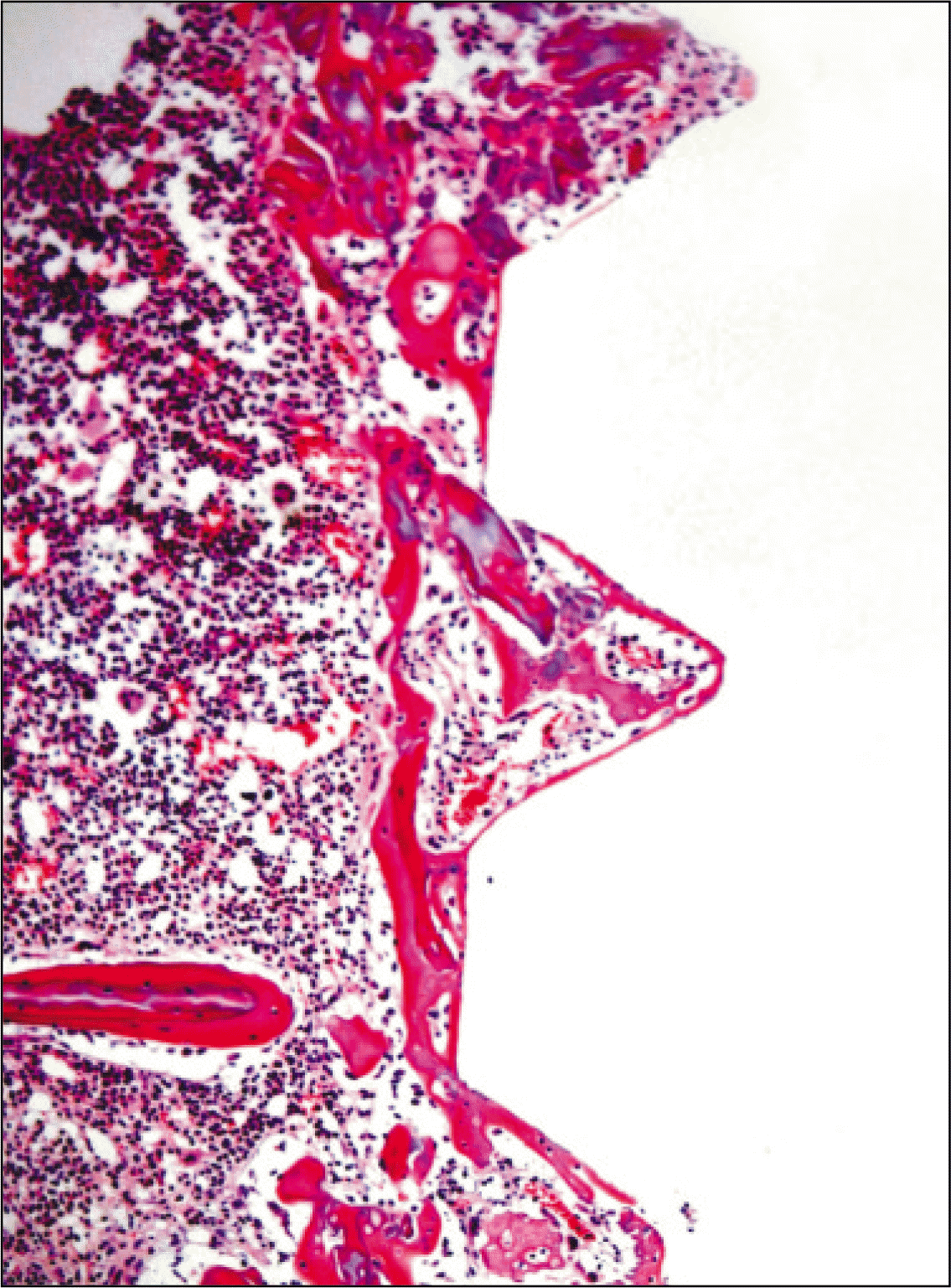 | Fig. 8.Microphotograph at 1 week after implantation in experimental rat.(H&E staining, original magnification ×100) |
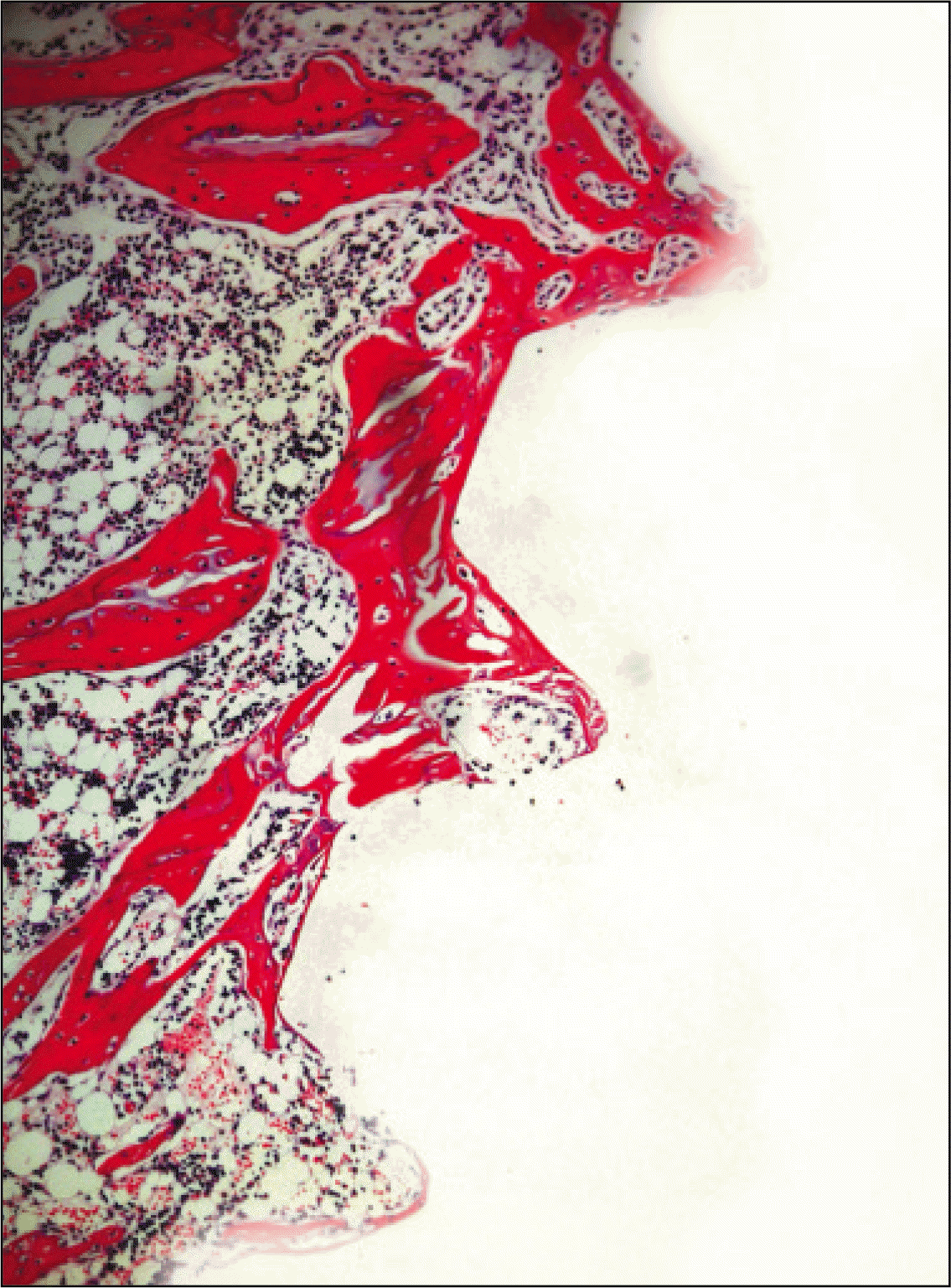 | Fig. 9.Microphotograph at 2 weeks after implantation in experimental rat.(H&E staining, original magnification ×100) |
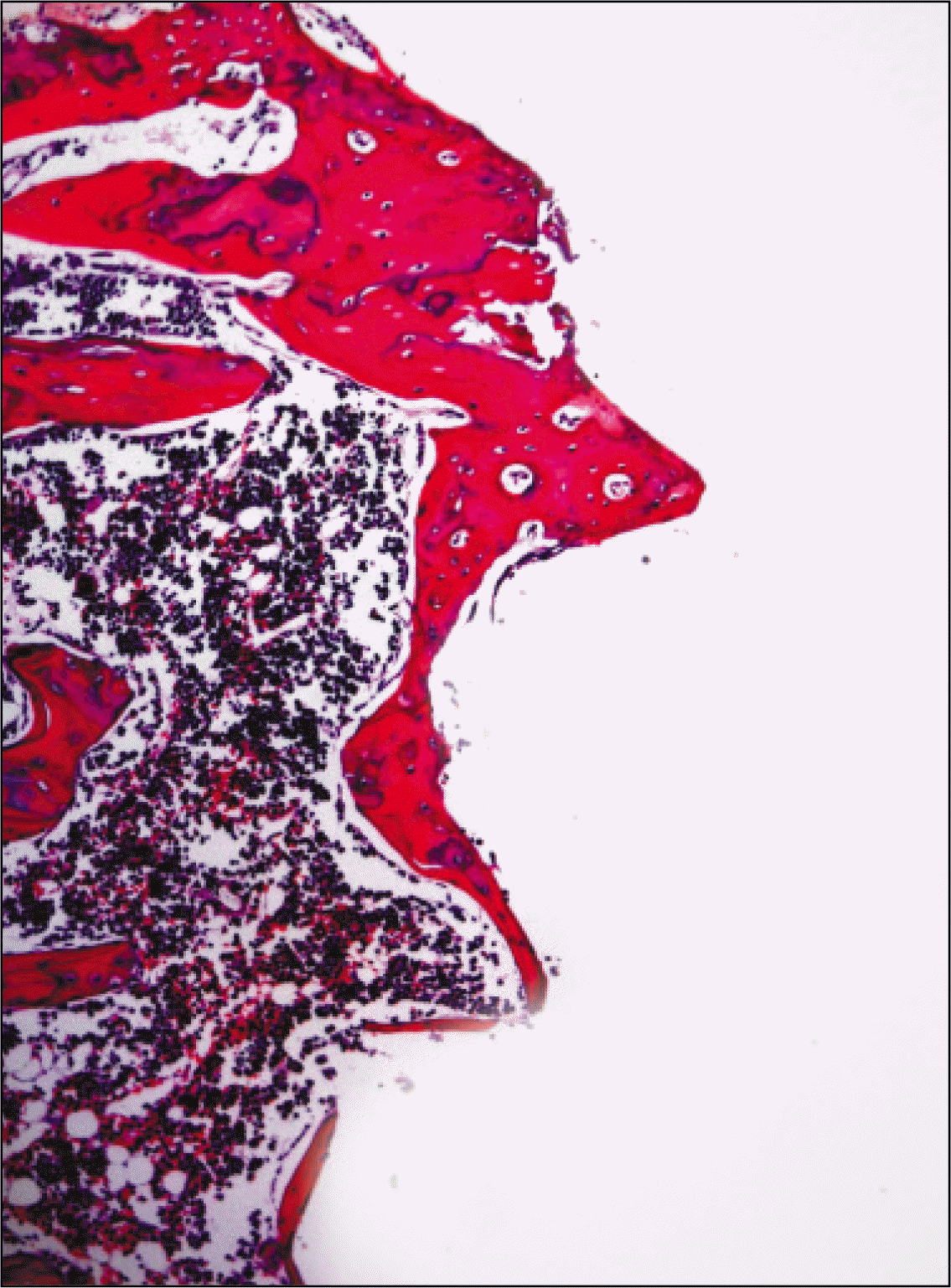 | Fig. 10.Microphotograph at 4 weeks after implantation in experimental rat.(H&E staining, original magnification ×100) |
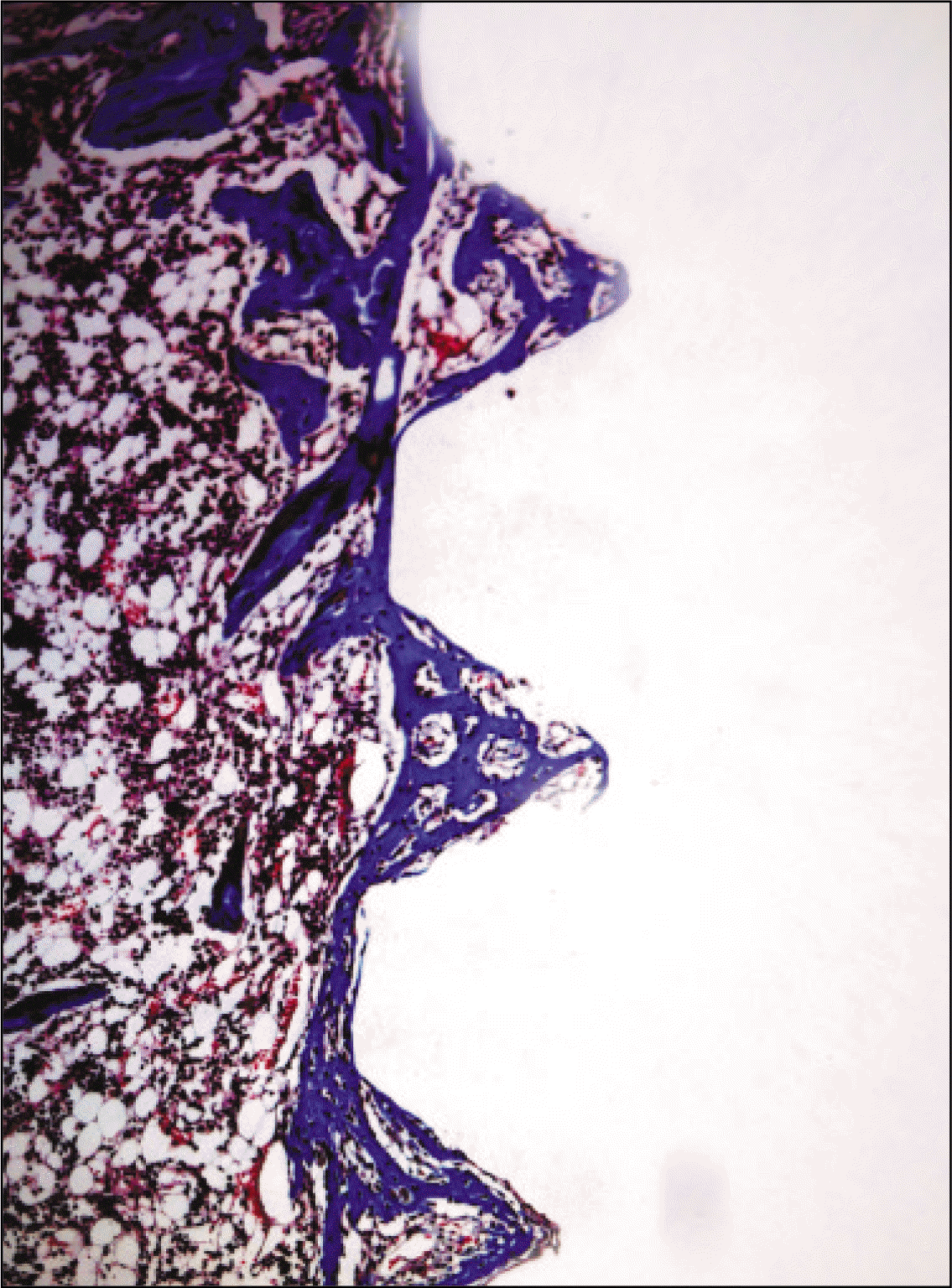 | Fig. 11.Microphotograph at 1 week after implantation in control rat. (Masson's trichrome stain. Original magnification ×100) |
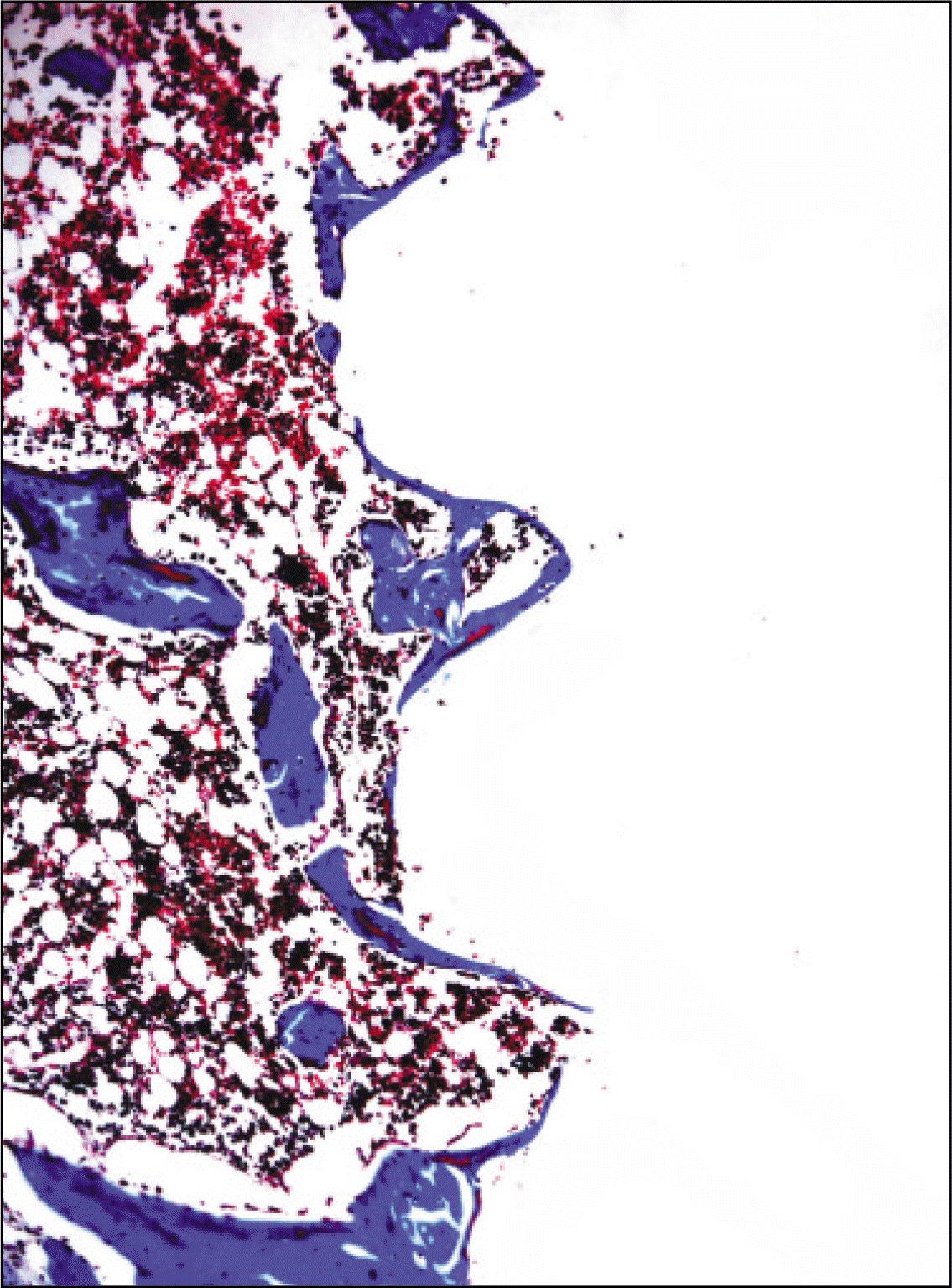 | Fig. 12.Microphotograph at 2 weeks after implantation in control rat. (Masson's trichrome stain. Original magnification ×100) |
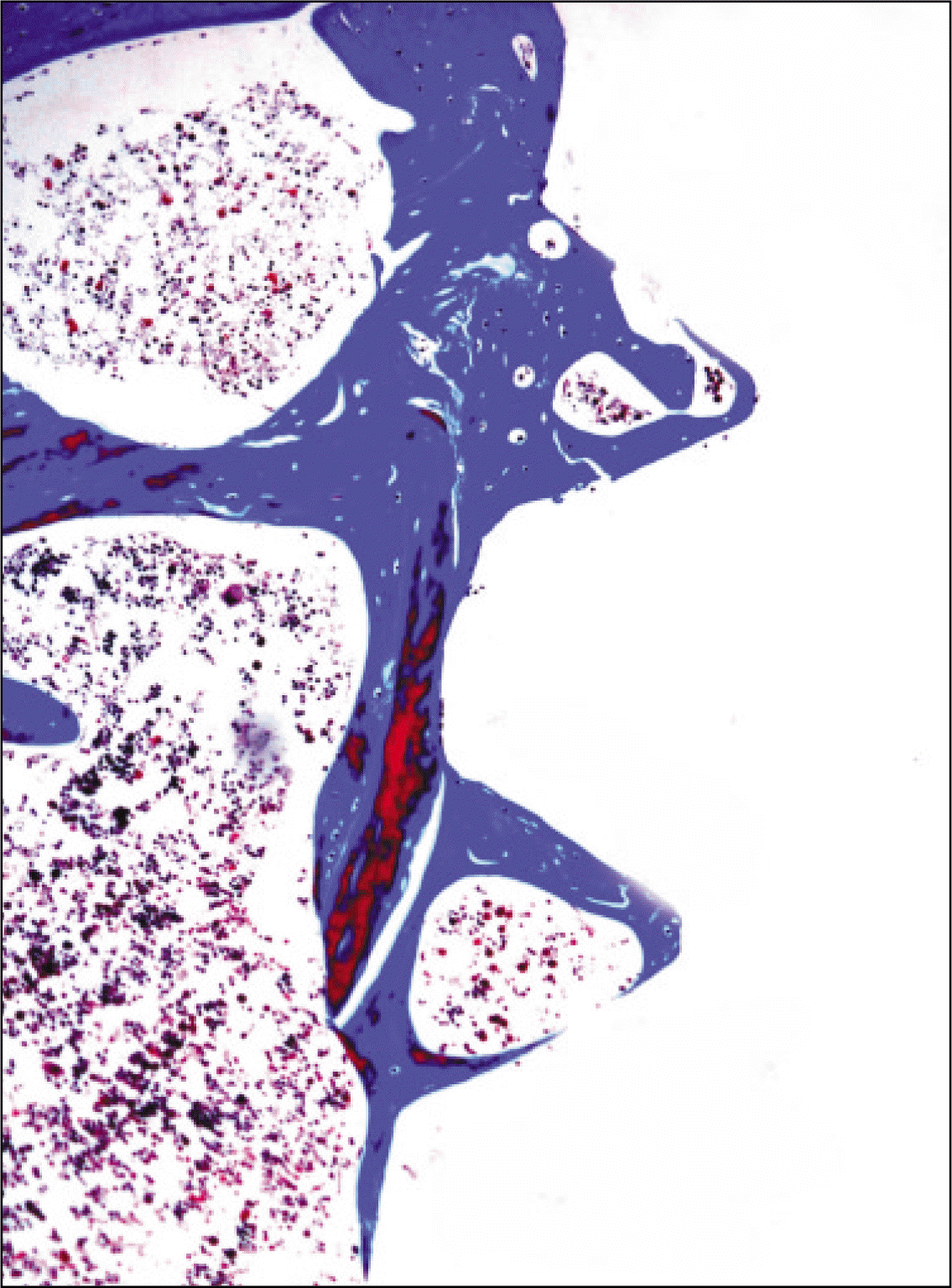 | Fig. 13.Microphotograph at 4 weeks after implantation in control rat. (Masson's trichrome stain. Original magnification ×100) |
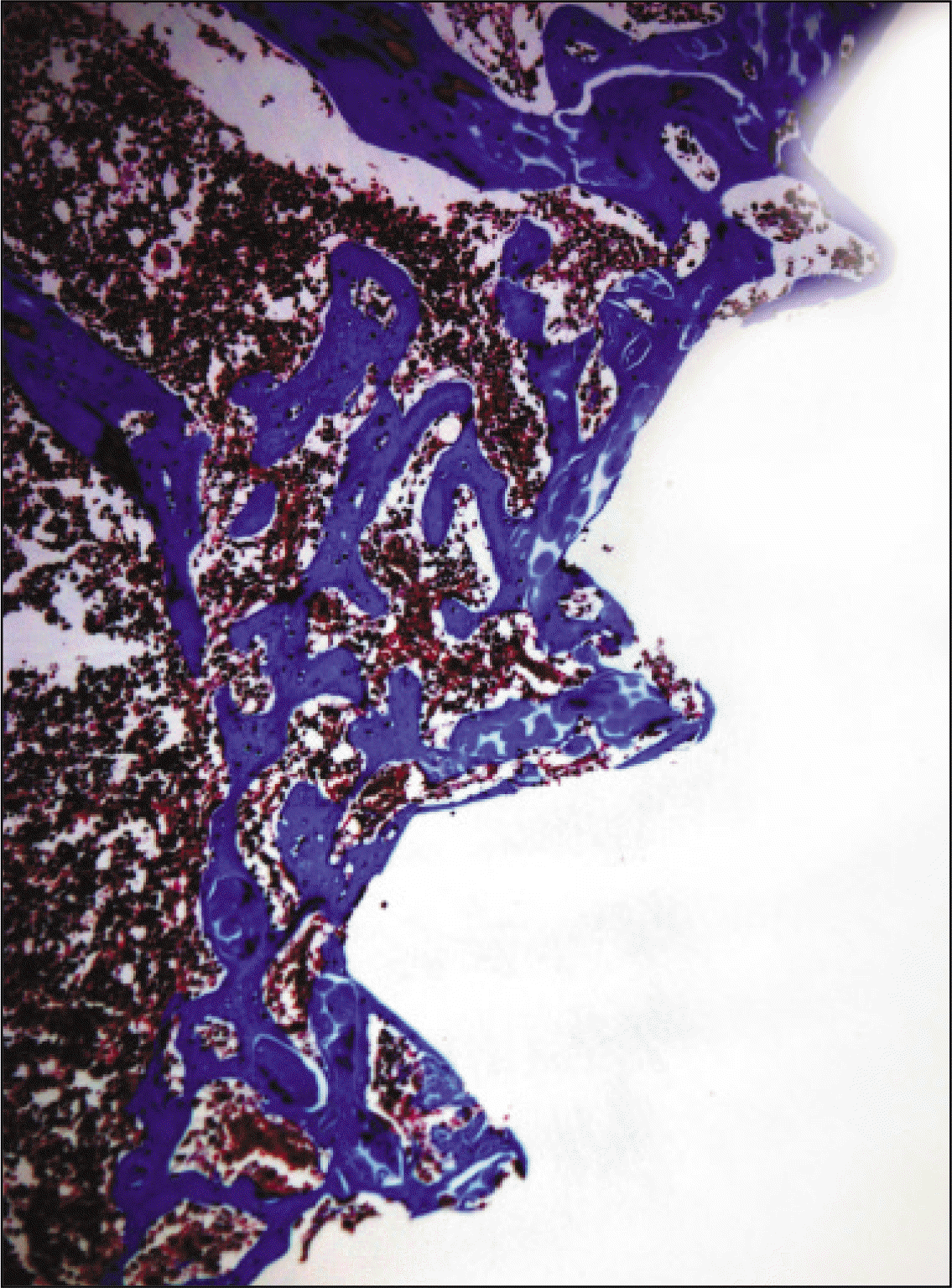 | Fig. 14.Microphotograph at 1 week after implantation in experimental rat. (Masson's trichrome stain. Original magnification ×100) |
 | Fig. 15.Microphotograph at 2 weeks after implantation in experimental rat. (Masson's trichrome stain. Original magnification ×100) |
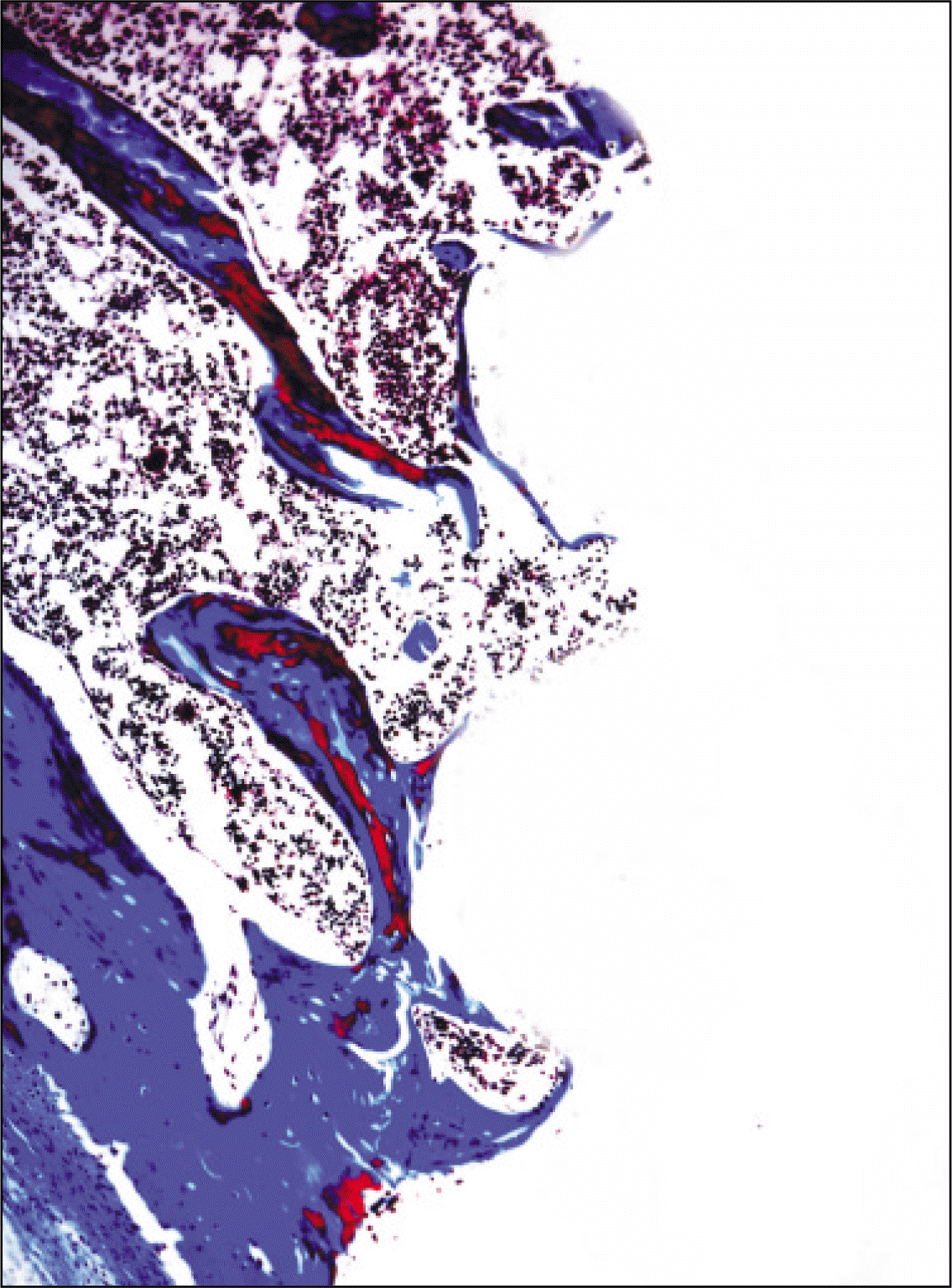 | Fig. 16.Microphotograph at 4 weeks after implantation in experimental rat. (Masson's trichrome stain. Original magnification ×100) |
 | Fig. 17.Osteoproteogrin antibody reaction of regenerating bone at 1 week after implantation on tibia of the control rat. ×200. |
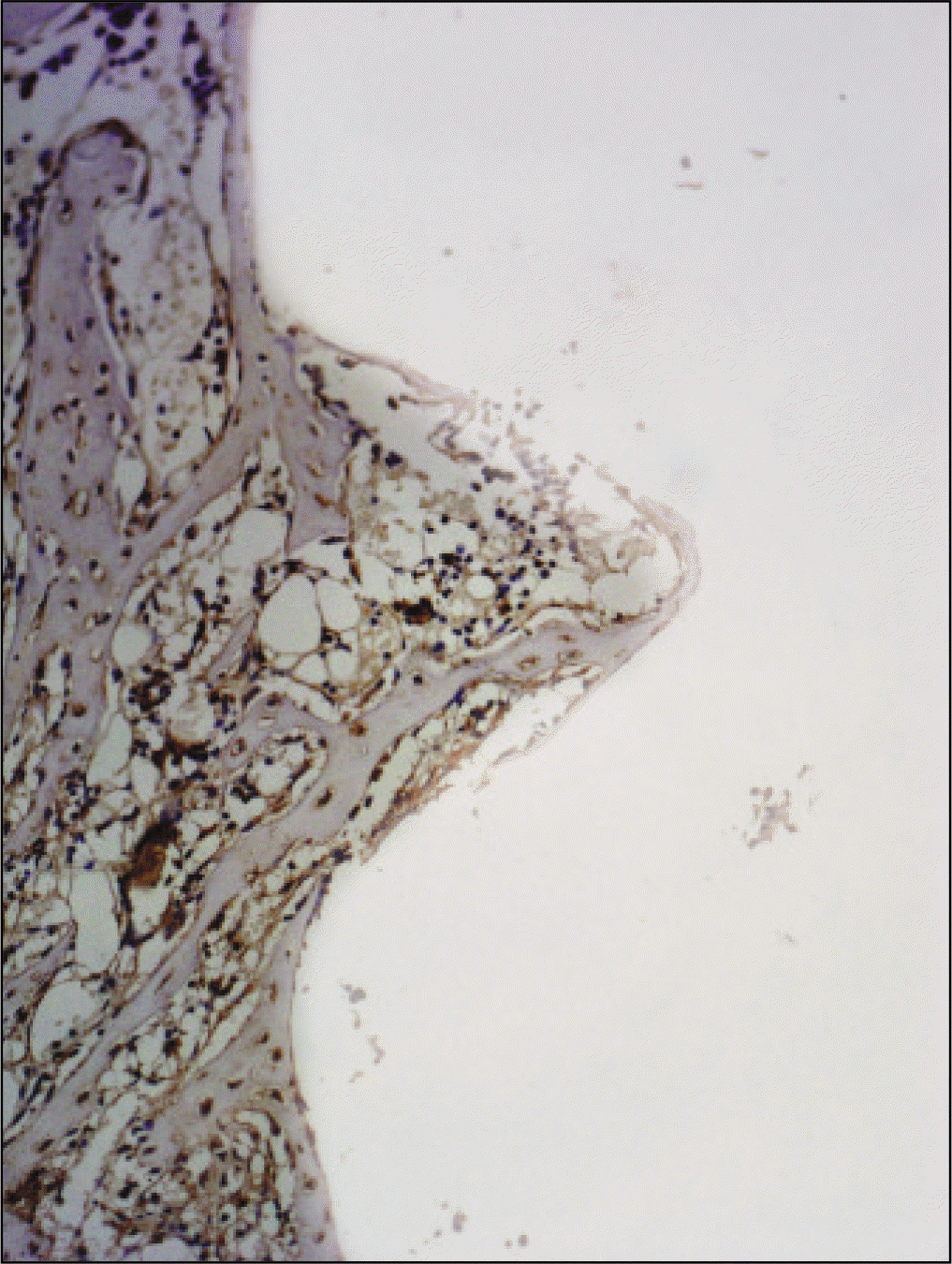 | Fig. 18.Osteoproteogrin antibody reaction of regenerating bone at 2 weeks after implantation on tibia of the control rat. ×200. |
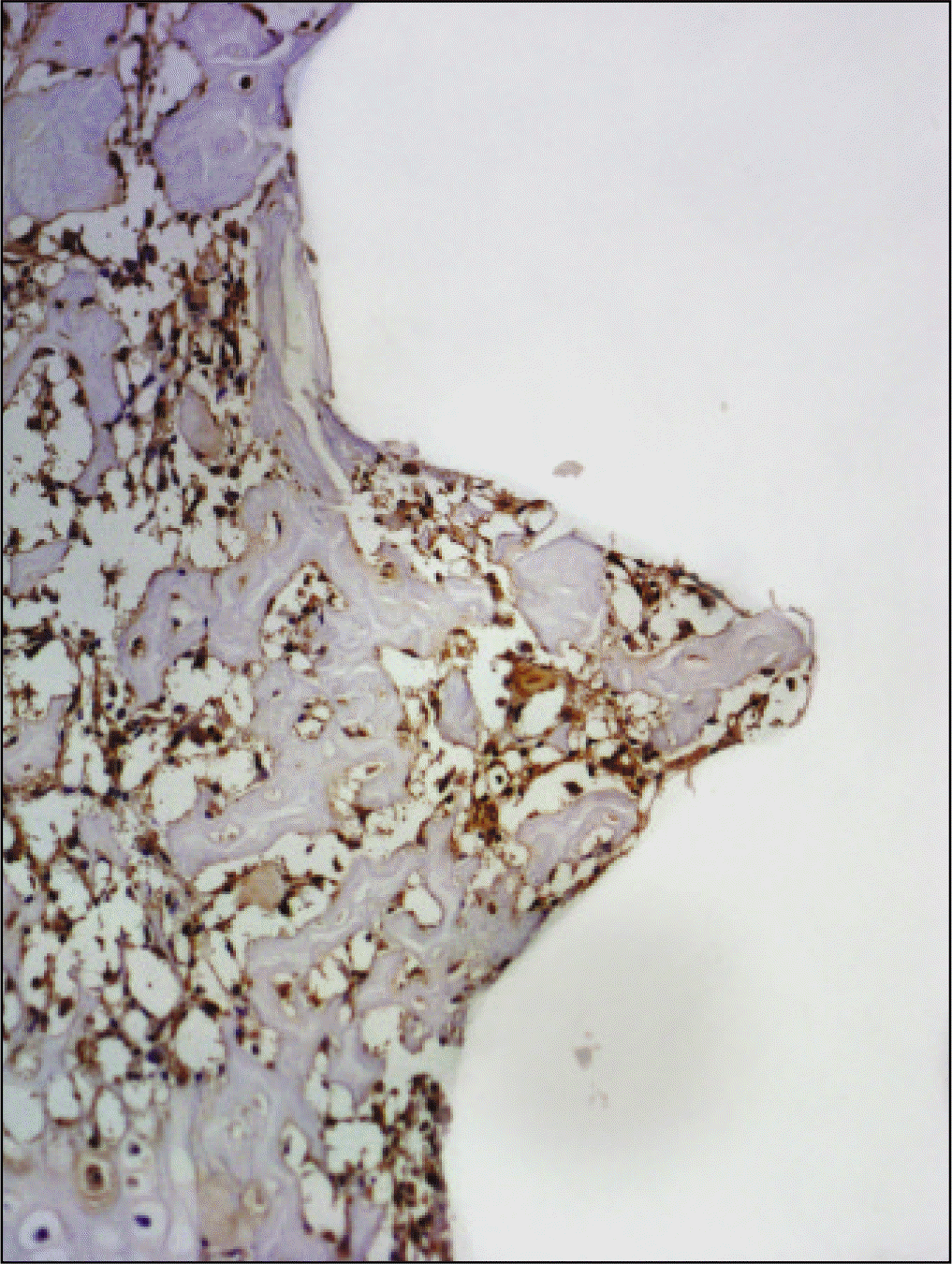 | Fig. 19.Osteoproteogrin antibody reaction of regenerating bone at 1 week after implantation on tibia of the Experimental rat. ×200. |
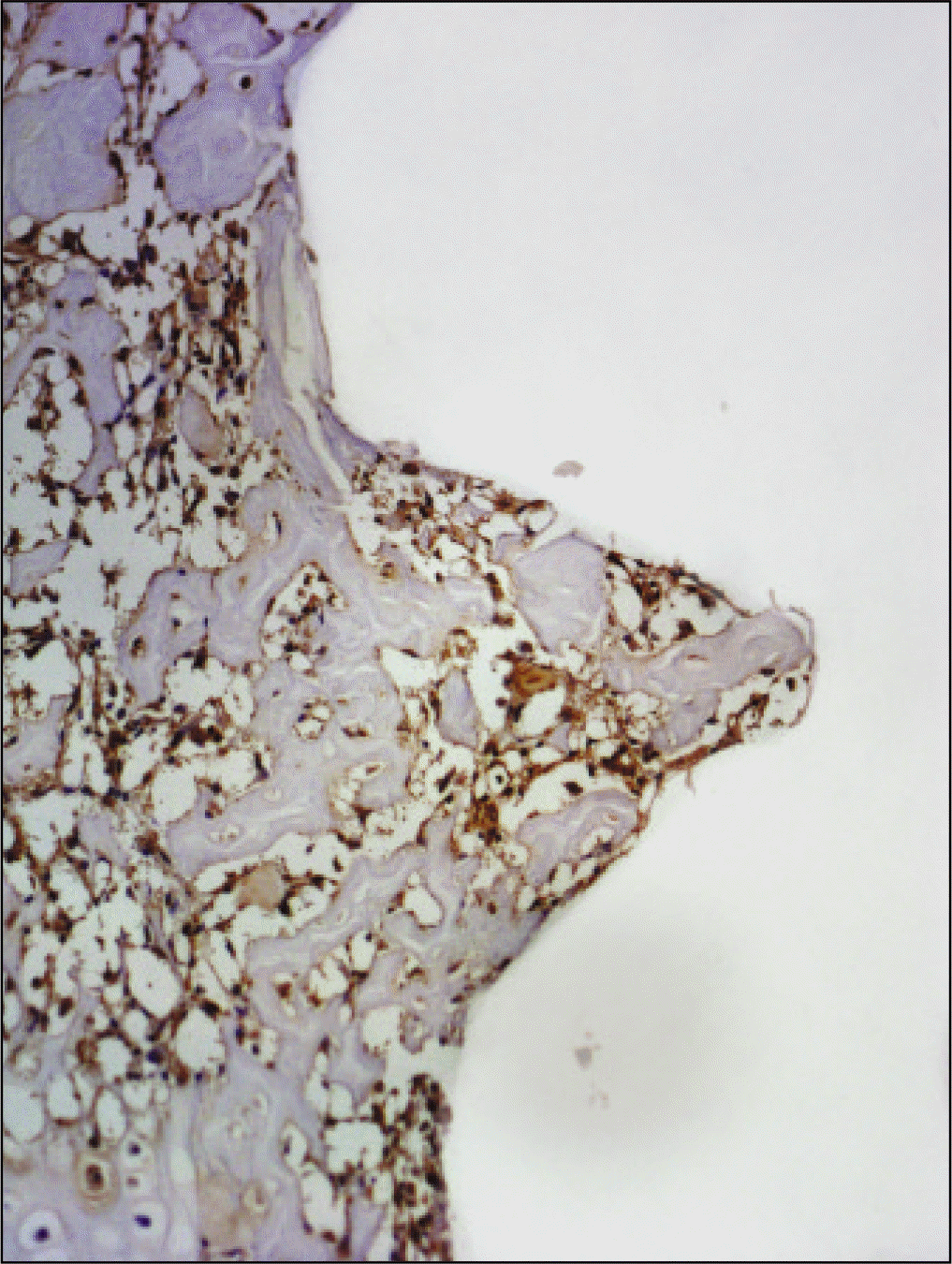 | Fig. 20.Osteoproteogrin antibody reaction of regenerating bone at 2 weeks after implantation on tibia of the Experimental rat. ×200. |
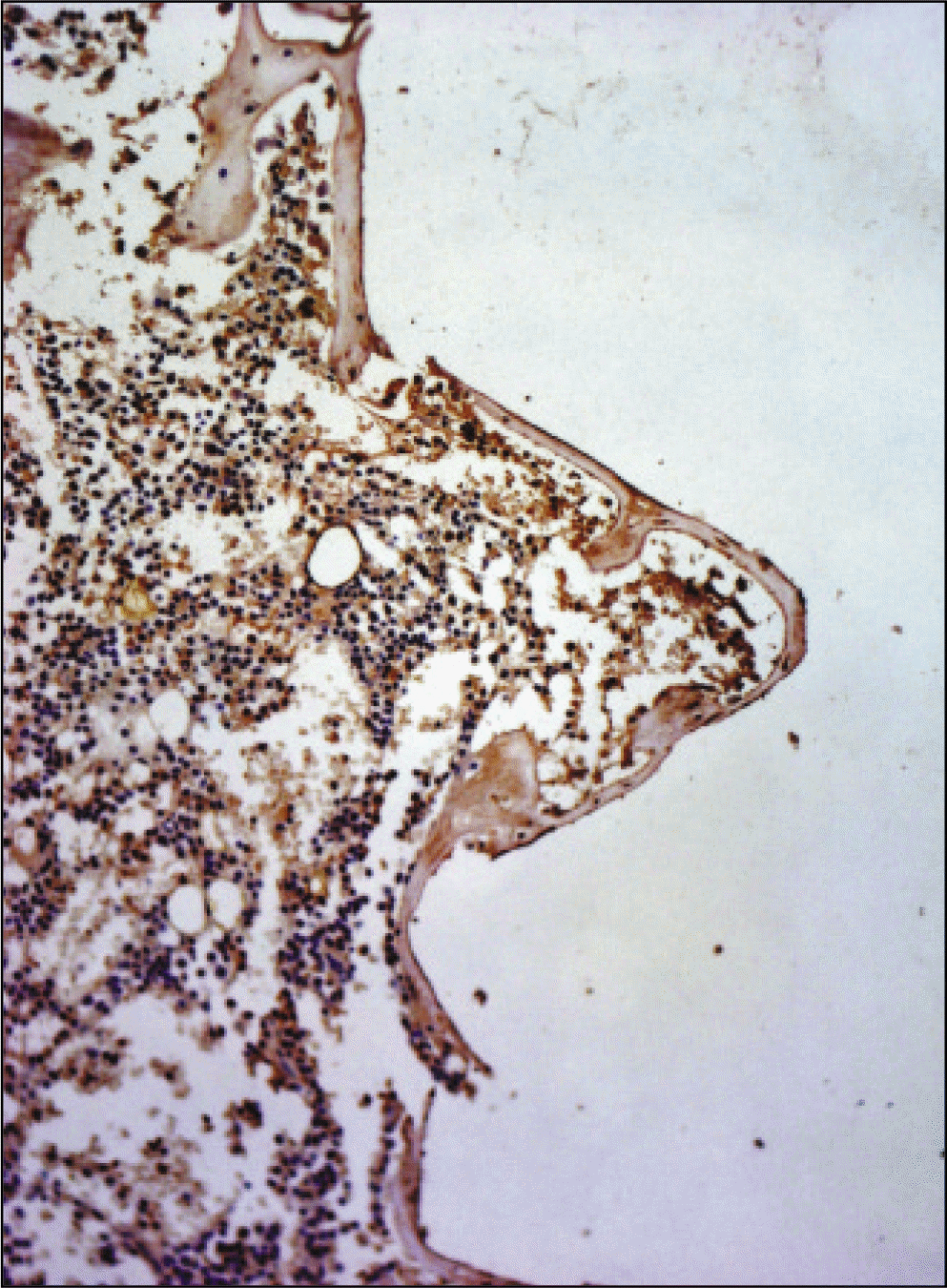 | Fig. 21.Receptor activator of nuclear factor kB ligand antibody reaction of regenerating bone at 1 weeks after implantation on tibia of the Control rat. ×200. |
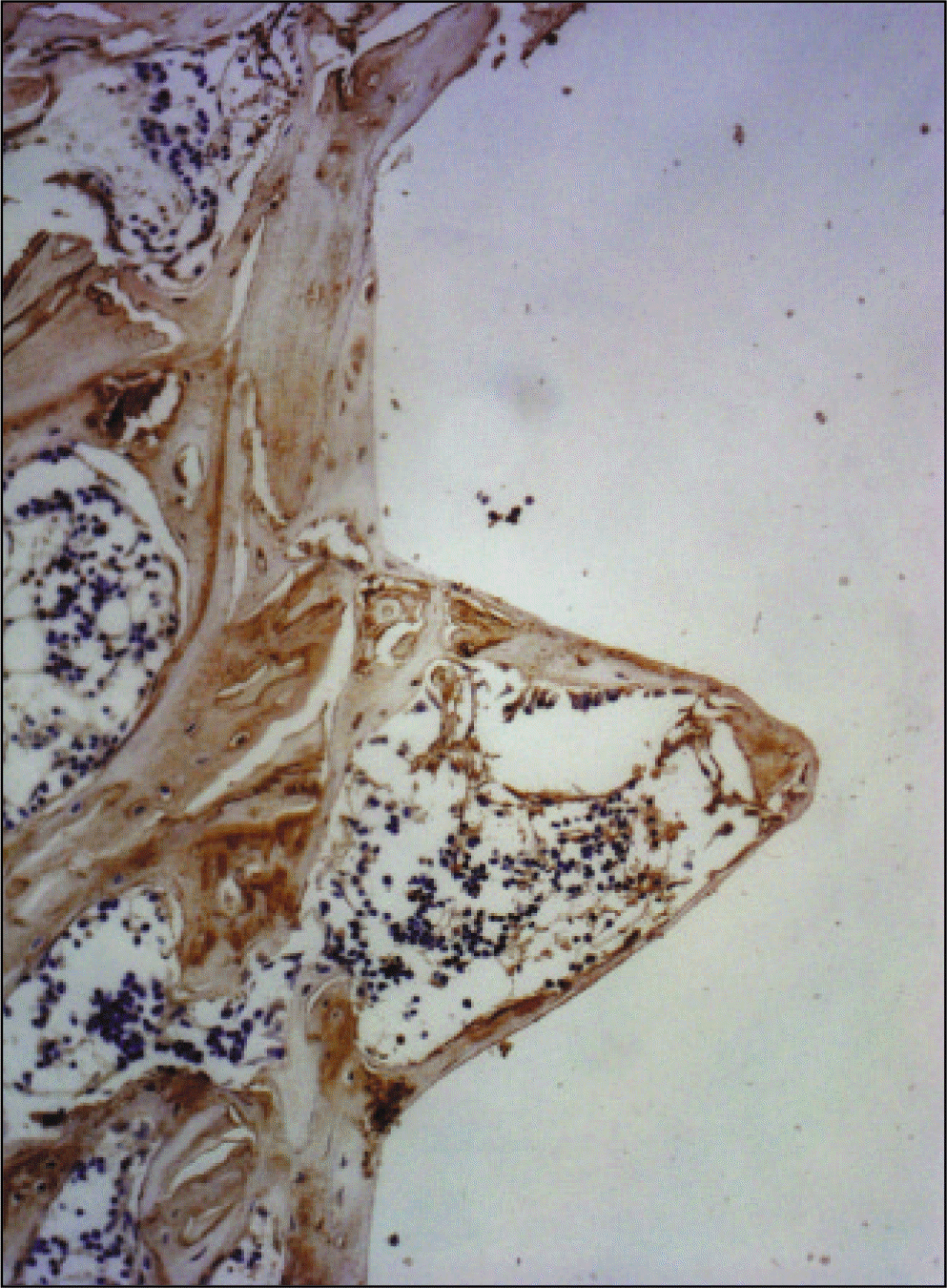 | Fig. 22.Receptor activator of nuclear factor kB ligand antibody reaction of regenerating bone at 2 weeks after implantation on tibia of the Control rat. ×200. |
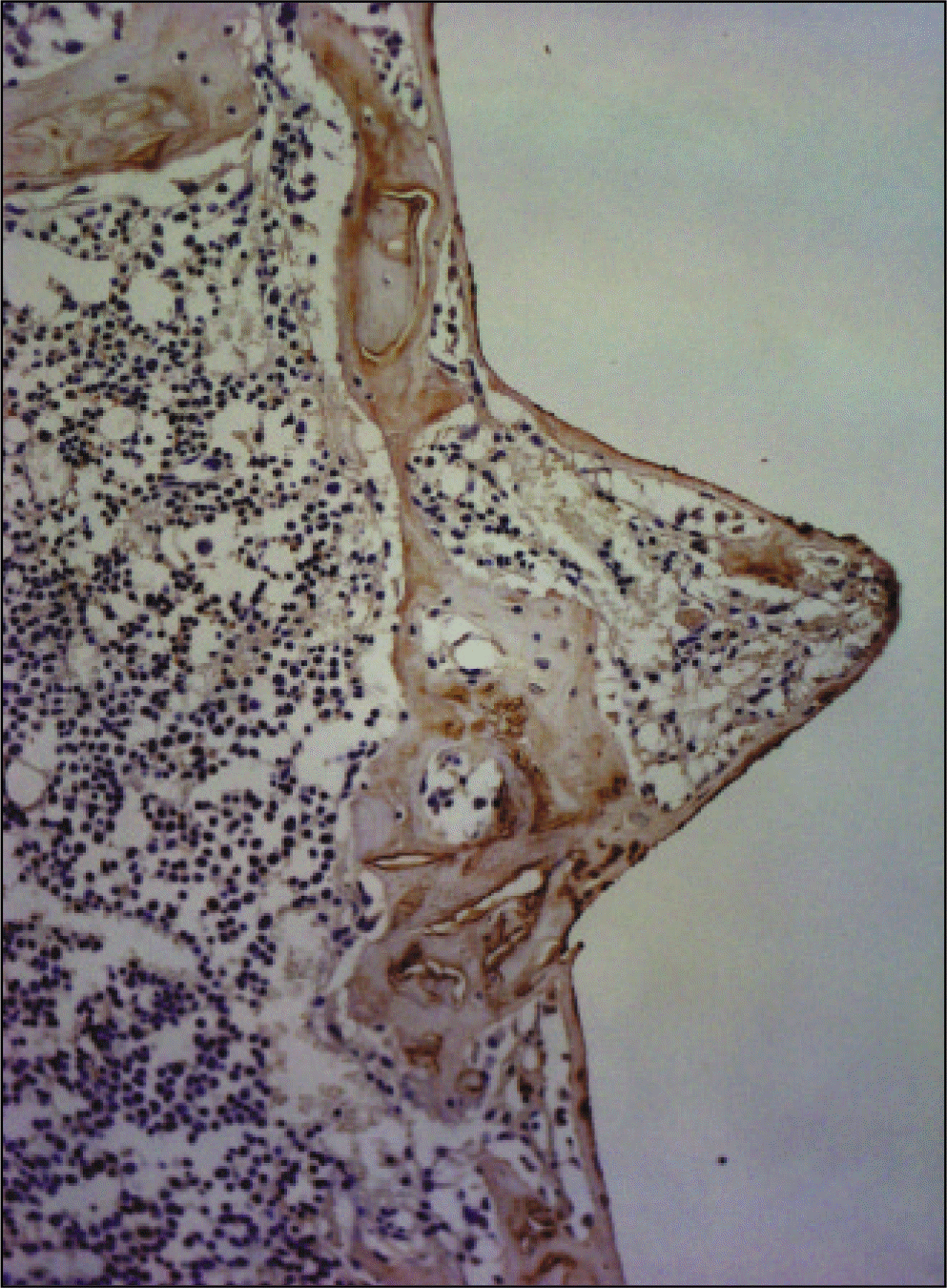 | Fig. 23.Receptor activator of nuclear factor kB ligand antibody reaction of regenerating bone at 1 weeks after implantation on tibia of the Experimental rat. ×200. |
 | Fig. 24.Receptor activator of nuclear factor kB ligand antibody reaction of regenerating bone at 2 weeks after implantation on tibia of the Experimental rat. ×200. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download