Abstract
Introduction
Bone resorption is a unique function of osteoclasts. Osteoclasts are a specialized macrophage polykaryon whose differentiation is regulated principally by macrophage colony-stimulating factors, receptor activator of nuclear factor κ B ligand (RANK) ligand, osteoprotegerin (OPG), and interleukins (IL). Reflecting the integrin-mediated signals, osteoclasts develop a specialized cytoskeleton that allows it to establish an isolated microenvironment between itself and the bone, wherein matrix degradation occurs by a process involving proton transport. The levels of IL-1, IL-6, OPG, and prostaglandin E2 (PGE2) expression were evaluated to study the correlations between dental implant teeth and the adjacent teeth.
Materials and Methods
The exudate of the gingival crevice acquired from dental implants, adjacent teeth, opposite teeth and contralateral teeth of 24 patients.
Results
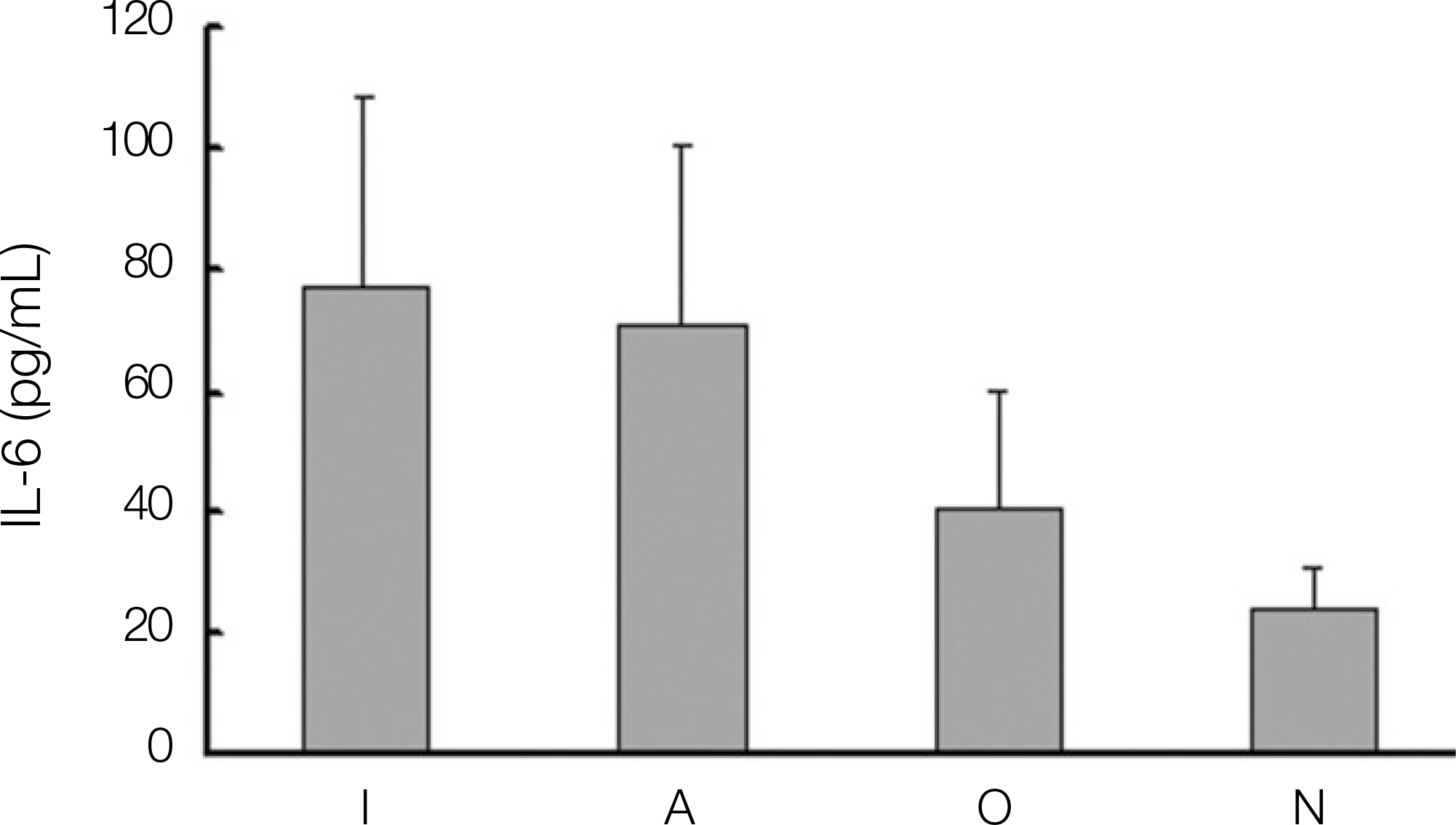
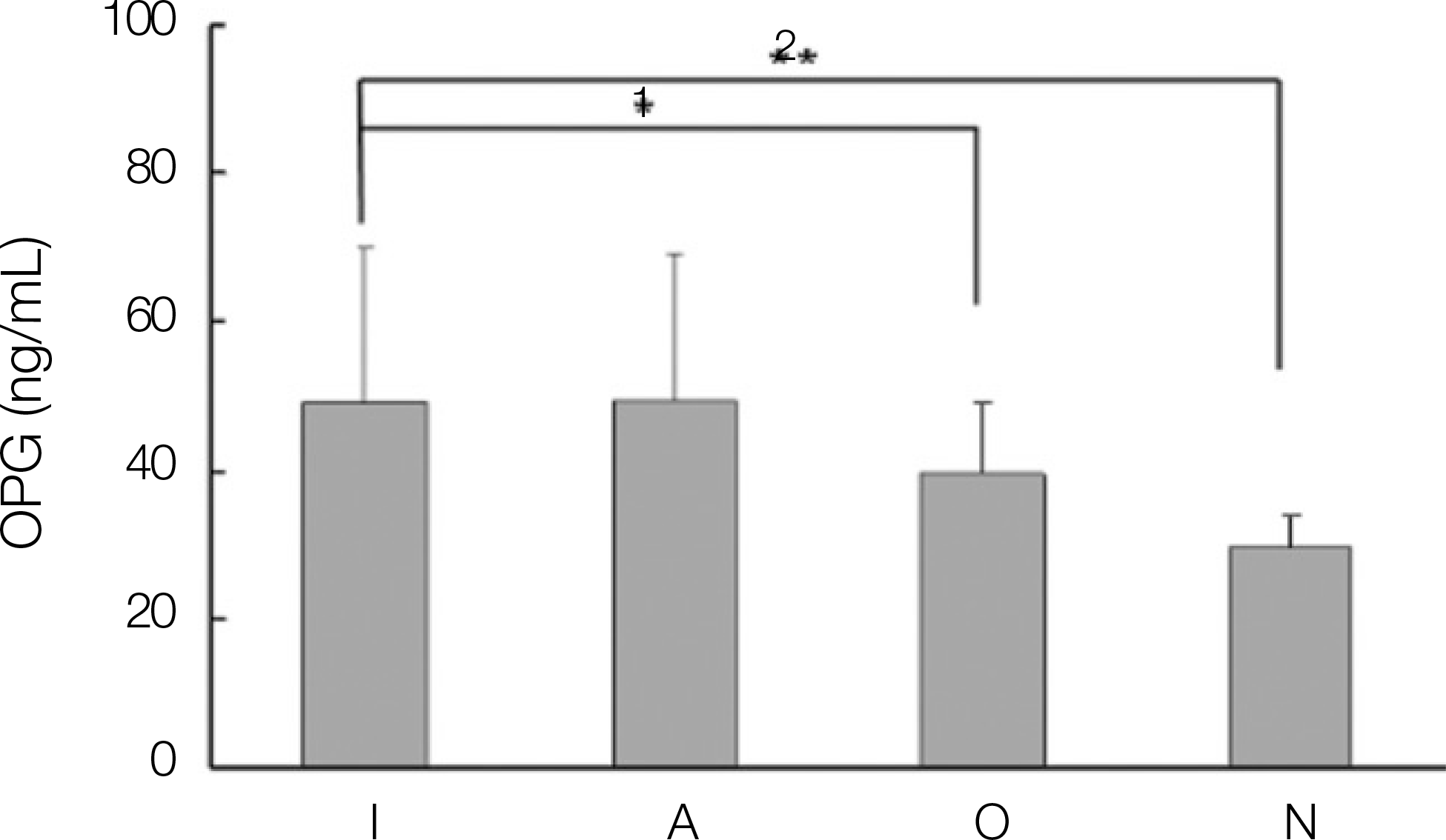
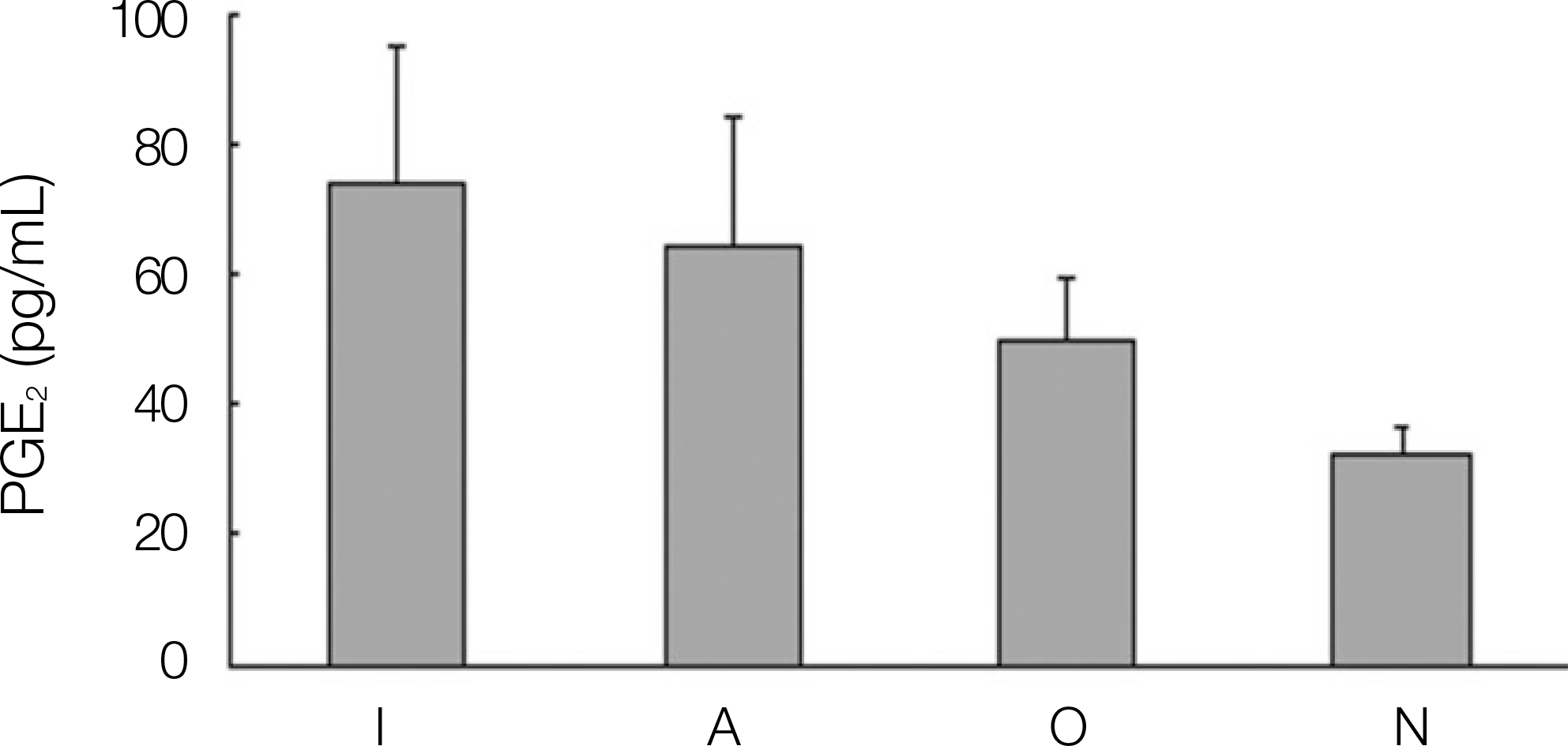
1. The levels of IL-1, IL-6, OPG and PGE2 expression in dental implant teeth were higher than those of the contralateral teeth. 2. IL-1 revealed a higher expression level in the adjacent teeth than in dental implant teeth. 3. The dental implant teeth and adjacent teeth did not show a remarkable difference in the level of IL-1 expression. 4. All the other cytokines were strongly expressed in the dental implant compared to the adjacent teeth.
References
1. Hernandez CJ, Beaupre ′ GS, Carter DR. A model of mechanobio-logic and metabolic influences on bone adaptation. J Rehabil Res Dev. 2000; 37:235–44.
3. Ducy P, Schinke T, Karsenty G. The Osteoblast: A Sophisticated Fibroblast under Central Surveillance. Science. 2000; 289:1501–4.

4. Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-κ B ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001; 79:243–53.
5. Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor κB ligand upregulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002; 17:210–20.

6. Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000; 15:2–12.

7. Grieve WG 3rd, Johnson GK, Moore RN, Reinhardt RA, DuBois LM. Prostaglandin E (PGE) and interleukin-1 β (IL-1 β) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1994; 105:369–74.
8. Rody WJ Jr, King GJ, Gu G. Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2001; 120:477–89.

9. Chung HJ. Collagenolytic activity of gingival crevicular fluid in progressive periodontitis. J Korean Acad Periodontol. 1996; 26:161–75.
10. Lim CD, Moon JK, Kim HS. The protein composition of gingival crevicular fluid and serum sampled from normal subjects. J Korean Acad Periodontol. 1994; 24:178–84.
11. Basdra EK, Komposch G. Osteoblast-like properties of human periodontal ligament cells: an in vitro analysis. Eur J Orthod. 1997; 19:615–21.

12. Shimizu N, Ogura N, Yamaguchi M, Goseki T, Shibata Y, Abiko Y, et al. Stimulation by interleukin-1 of interleukin-6 production by human periodontal ligament cells. Arch Oral Biol. 1992; 37:743–8.

13. Okada N, Kobayashi M, Mugikura K, Okamatsu Y, Hanazawa S, Kitano S, et al. Interleukin-6 production in human fibroblasts derived from periodontal tissues is differentially regulated by cytokines and a glucocorticoid. J Periodontal Res. 1997; 32:559–69.

14. Udagawa N, Takahashi N, Katagiri T, Tamura T, Wada S, Findlay DM, et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med. 1995; 182:1461–8.

16. Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995; 332:305–11.
Fig. 1.
Expression of interleukin-1 α(IL-1 α) in dental implant patients. (I: dental implant teeth, A: adjacent teeth to the dental implant, O: opposite, N: normal teeth)
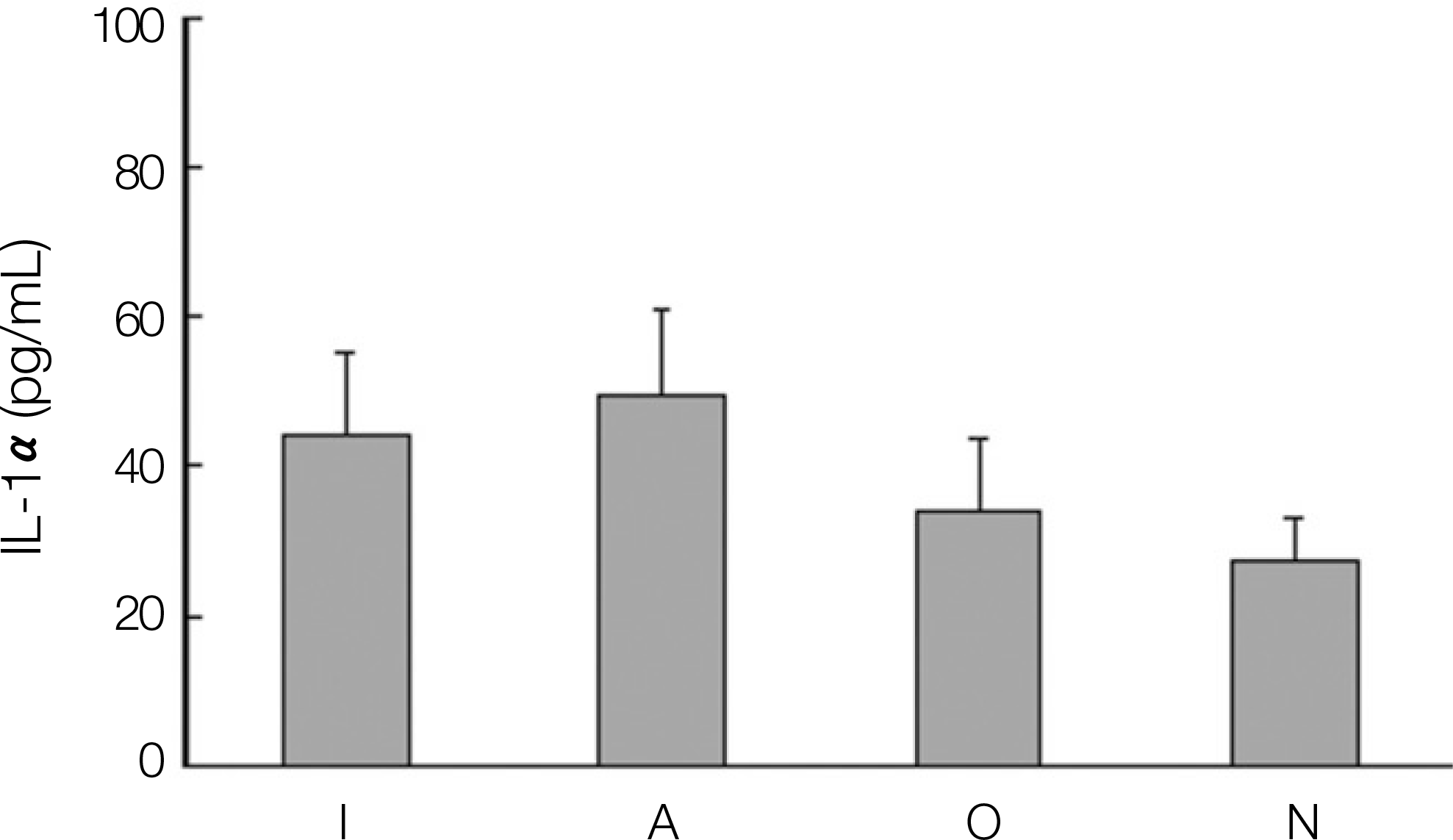
Fig. 2.
Expression of interleukin-1 β(IL-1 β) in dental implant patients. (I: dental implant teeth, A: adjacent teeth to the dental implant, O: opposite, N: normal teeth. 1: P<0.01, 2: P<0.01)
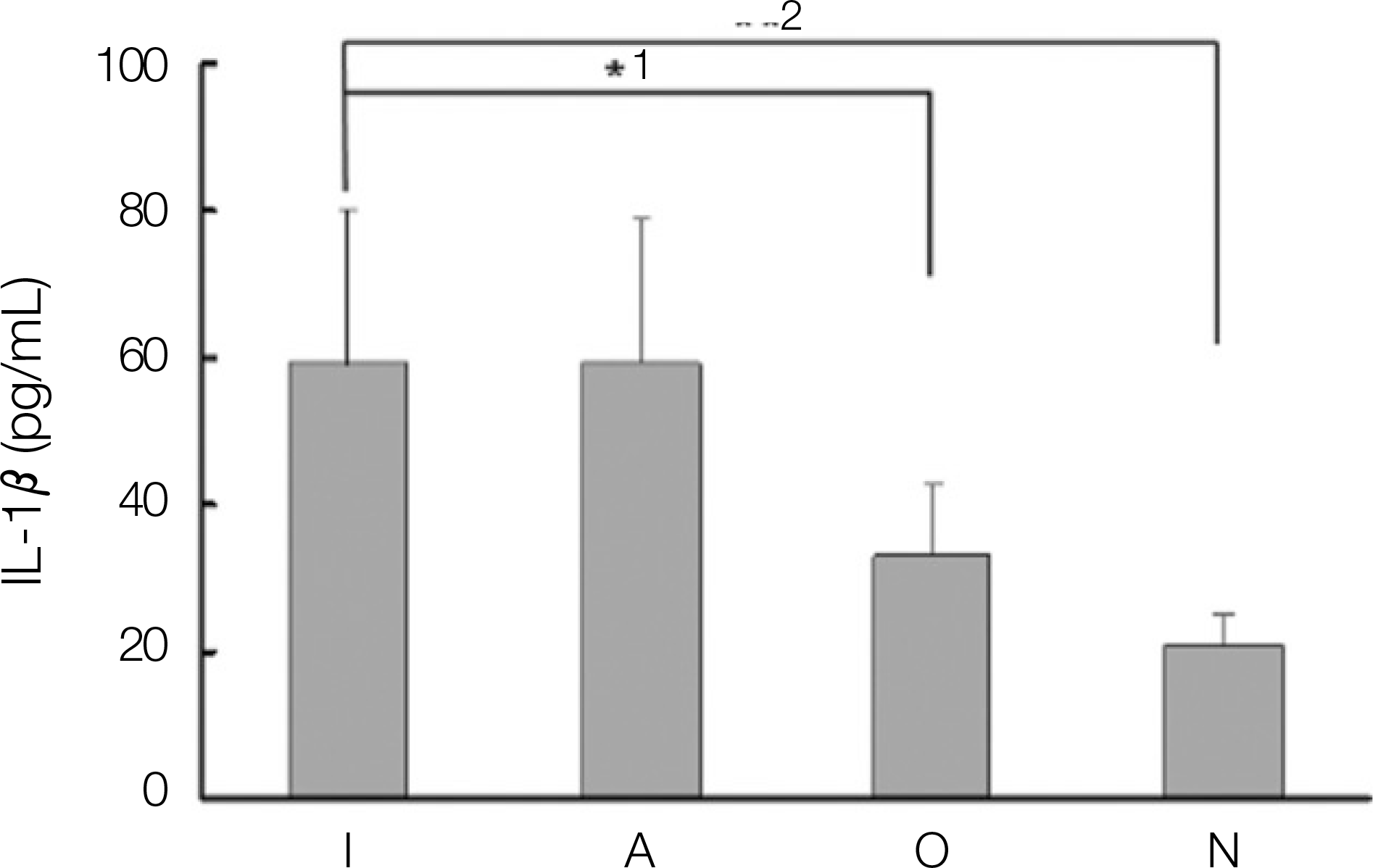
Fig. 3.
Expression of interleukin-6 (IL-6) in dental implant patients. (I: dental implant teeth, A: adjacent teeth to the dental implant, O: opposite, N: normal teeth)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download