Abstract
Introduction
Guided bone regeneration (GBR) is a common procedure for the treatment of bone defects and bone augmentation. The non-resorbable barriers are well-documented barriers for GBR because of their stability and malleability. However, few GBR studies have focused on the different types of non-resorbable barriers. Therefore, this study examined the clinical results of different non-resorbable barriers for GBR; expanded polytetrafluoroethylene (e-PTFE) (TR-Gore Tex, Flagstaff, AZ, USA), and high-density polytetrafluoroethylene (d-PTFE) (Cytoplast membrane, Oraltronics, Bremen, Germany).
Materials and Methods
The analysis was performed on patients treated with GBR and implant placement from January 2007 to October 2007 in the department of the Seoul National University Bundang Hospital. The patients were divided into two groups based on the type of non-resorbable barrier used, and the amount of bone regeneration, marginal bone resorption after prosthetics, implant survival rate and surgical complication in both groups were evaluated.
Results
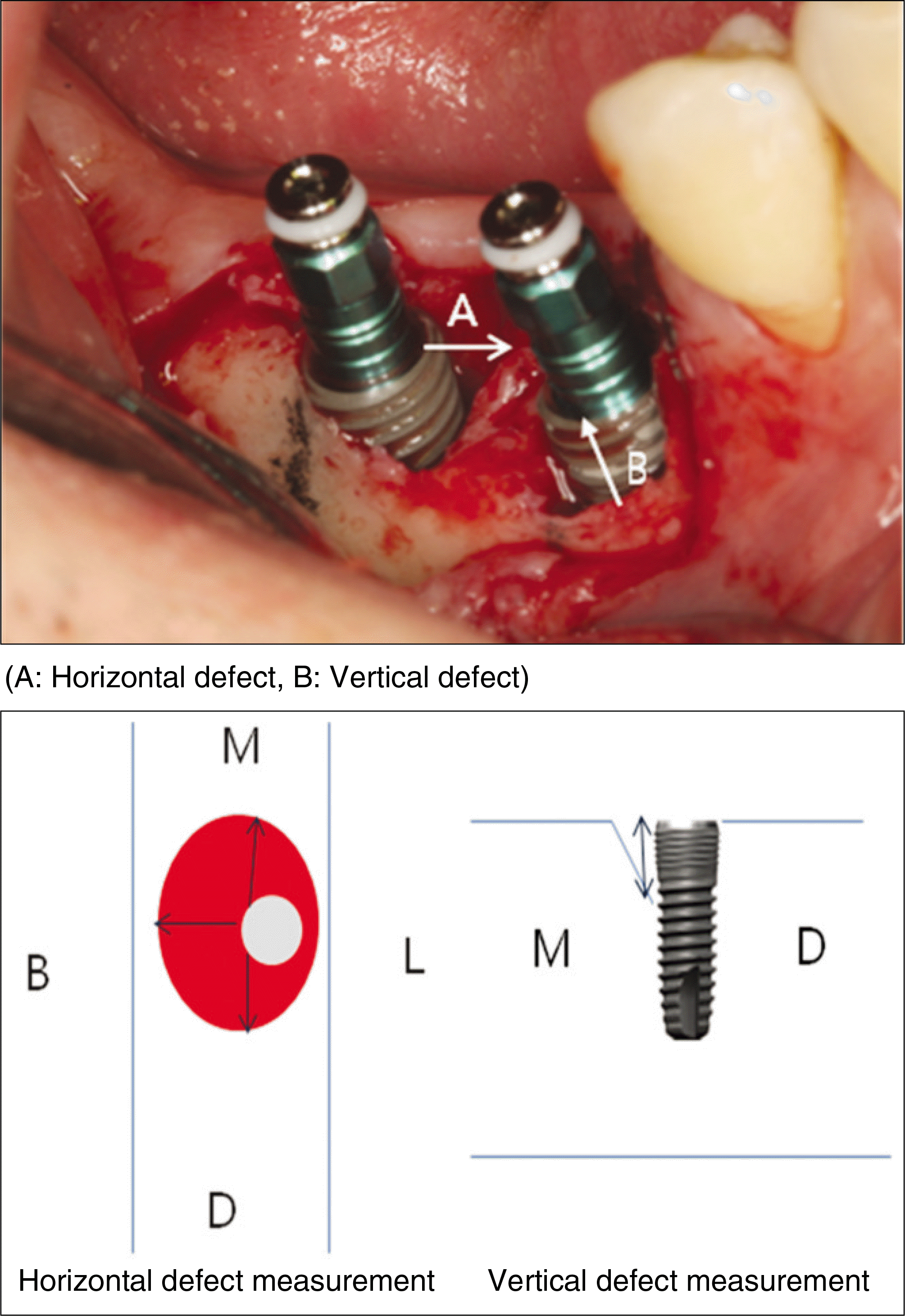
The implants in both groups showed high survival rates, and the implant-supported prostheses functioned stably during the follow-up period. During the second surgery of the implant, all horizontal defects were filled with new bone, and there was no significant difference in the amount of vertical bone defect.
References
1. Kim YK, Kim SG, Lim SC, Lee HJ, Yun PY. A clinical study on the bone formation using a demineralized bone matrix and resorbable membrane. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:e6–11.
2. Bernabe ′PF, Gomes-Filho JE, Cintra LT, Moretto MJ, Lodi CS, Nery MJ, et al. Histologic evaluation of the use of membrane, bone graft, and MTA in apical surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:309–14.
3. Kim YK, Yun PY, Kim SG, Oh DS. In vitro scanning electron microscopic comparison of inner surface of exposed and unexposed nonresorbable membranes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e5–e11.

4. Rominger JW, Triplett RG. The use of guided tissue regeneration to improve implant osseointegration. J Oral Maxillofac Surg. 1994; 52:106–12.

5. Barber HD, Lignelli J, Smith BM, Bartee BK. Using a dense PTFE membrane without primary closure to achieve bone and tissue regeneration. J Oral Maxillofac Surg. 2007; 65:748–52.

6. Dahlin C, Sennerby L, Lekholm U, Linde A, Nyman S. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1989; 4:19–25.
7. Aichelmann-Reidy ME, Yukna RA, Evans GH, Nasr HF, Mayer ET. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001; 72:998–1005.

8. Eickholz P, Kim TS, Holle R. Regenerative periodontal surgery with nonresorbable and biodegrabable barriers: results after 24 months. J Clin Periodontol. 1998; 25:666–76.
9. Teparat T, Solt CW, Claman LJ, Beck FM. Clinical comparison of bioabsorbable barriers with non resorbable barriers in guided tissue regeneration in the treatment of human intrabony defects. J Periodontol. 1998; 69:632–41.
10. Dahlin C, Lekholm U, Becker W, Becker B, Higuchi K, Callens A, et al. Treatment of fenestration and dehiscence bone defects around oral implant using guided tissue regeneration technique: a prospective multicenter study. Int J Oral Maxillofac Implants. 1995; 10:312–8.
11. Buser D, Bra ¨ gger U, Lang NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implant Res. 1990; 1:22–32.

12. Herr Y. Periodontology-based implantology. Seoul: Myungmoon Publishing;2006.
13. Bartee BK. Evaluation of new polytetrafluoroethylene guided tissue regeneration membrane in healing extraction sites. Compend Contin Educ Dent. 1998 Dec; 19(12):1256–8. 1260, 1262–4.
14. Pretzl B, Kim TS, Holle R, Eickholz P. Long-term results of guided tissue regeneration therapy with nonresorbable and bioabsorbable barriers. IV. A case series of infrabony defects after 10 years. J Periodontol. 2008; 79:1491–9.

15. Bartee BK. The use of high-density polytetrafluoroethylene membrane to treat osseous defects: clinical reports. Implant Dent. 1995; 4:21–6.
16. Bartee BK, Carr JA. Evaluation of high-density polytetrafluoroethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regeration in the rat mandible. J Oral Implantol. 1995; 21:88–95.
17. Barber HD, Lignelli J, Smith BM, Bartee BK. Using dense PTFE membrane without primary closure to achieve bone and tissue regeneration. J Oral Maxillofac Surg. 2007; 65:748–52.
18. Piattelli A, Scarano A, Paolantonio M. Bone formation inside the material interstices of e-PTFE membranes: a light microscopical and histochemical study in man. Biomaterials. 1996; 17:1725–31.

19. Strietzel FP, Khongkhunthian P, Khattiya R, Patchanee P, Reichart PA. Healing pattern of bone defects covered by different membrane types- A histologic study in the porcine mandible. J Biomed Mater Res B Appl Biomater. 2006; 78:35–46.
20. Walters SP, Greenwell H, Hill M, Drisko C, Pickman K, Scheetz JP. Comparison of porous and non porous teflon membranes plus a xenograft in the treatment of vertical osseous defects: a clinical reentry study. J Periodontol. 2003; 74:1161–8.
Table 1.
Postoperative complications
| Complications | Group 1 | Group 2 |
|---|---|---|
| Wound dehiscence | 4 | 6 |
| Wound dehiscence+Ecchymosis | 1 | 0 |
Table 2.
Primary bone defects (Mean (SD))
| Group 1 | Group 2 | |
|---|---|---|
| Vertical bone defect (mm) | 2.29 (4.54) | 3.24 (4.9) |
| Horizontal bone defect (mm) | 2.36 (0.8) | 1.4 (0.84) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download