Abstract
Introduction
This study evaluated the bone regenerative effect of silk fibroin mixed with platelet-rich fibrin (PRF) of a bone defect in rabbits.
Materials and Methods
Ten New Zealand white rabbits were used for this study and bilateral round shaped defects were formed in the parietal bone (diameter: 8.0 mm). The silk fibroin mixed with PRF was grafted into the right parietal bone (experimental group). The left side (control group) was grafted only PRF. The animals were sacrificed at 4 weeks and 8 weeks. A micro-computerized tomography (μ CT) of each specimen was taken. Subsequently, the specimens were decalcified and stained for histological analysis.
Results
The average value of plane film analysis was higher in the experimental group than in the control group at 4 weeks and 8weeks after surgery. However, the difference was not statistically significant.(P>0.05) The tissue mineral density (TMD) in the experimental group at 4 weeks after surgery was significantly higher than the control group.(P<0.05)
Go to : 
References
1. Buser D, Dula K, Hess D, Hirt HP, Belser UC. Localized ridge augmentation with autografts and barrier membranes. Periodontol 2000. 1999; 19:151–63.

2. Laurie SW, Kaban LB, Mulliken JB, Murray JE. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984; 73:933–8.

3. Sommers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989; 71:677–80.
5. Friedlaender GE, Horowitz MC. Immune responses to osteochondral allografts: nature and significance. Orthopedics. 1992; 15:1171–5.

6. Carlson ER, Marx RE, Buck BE. The potential for HIV transmission through allogeneic bone. A review of risk and safety. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 80:17–23.
7. Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002; 18:27–33.

8. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001; 10:225–8.

9. Rodriguez A, Anastassov GE, Lee H, Buchbinder D, Wettan H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J Oral Maxillofac Surg. 2003; 61:157–63.

10. Tayapongsak P, O'Brien DA, Monteiro CB, Arceo-Diaz LY. Autologous fibrin adhesive in mandibular reconstruction with particulate cancellous bone and marrow. J Oral Maxillofac Surg. 1994; 52:161–5.

11. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich Plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:638–46.
12. Robiony M, Polini F, Costa F, Politi M. Osteogenesis distraction and platelet-rich plasma for bone restoration of the severely atrophic mandible: preliminary results. J Oral Maxillofac Surg. 2002; 60:630–5.

13. Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:299–303.

14. Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003; 24:401–16.

15. Huang J, Wong C, George A, Kaplan DL. The effect of genetically engineered spider silk-dentin matrix protein 1 chimeric protein on hydroxyapatite nucleation. Biomaterials. 2007; 28:2358–67.

16. Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, et al. Silk implants for the healing of critical size bone defects. Bone. 2005; 37:688–98.

17. Hirano Y, Mooney DJ. Peptide and protein presenting materials for tissue engineering. Adv Mater. 2004; 16:17–25.

18. Dal Pra I, Freddi G, Minic J, Chiarini A, Armato U. De novo engineering of reticular connective tissue in vivo by silk fibroin nonwoven materials. Biomaterials. 2005; 26:1987–99.

19. Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006; 27:1088–94.

20. Chan CK, Kumar TS, Liao S, Murugan R, Ngiam M, Ramakrishnan S. Biomimetic nanocomposites for bone graft applications. Nanomedicine (Lond). 2006; 1:177–88.

21. Zhao J, Zhang Z, Wang S, Sun X, Zhang X, Chen J, et al. Apatite-coated silk fibroin scaffolds to healing mandibular border defects in canines. Bone. 2009; 45:517–27.

22. Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006; 27:3115–24.

Go to : 
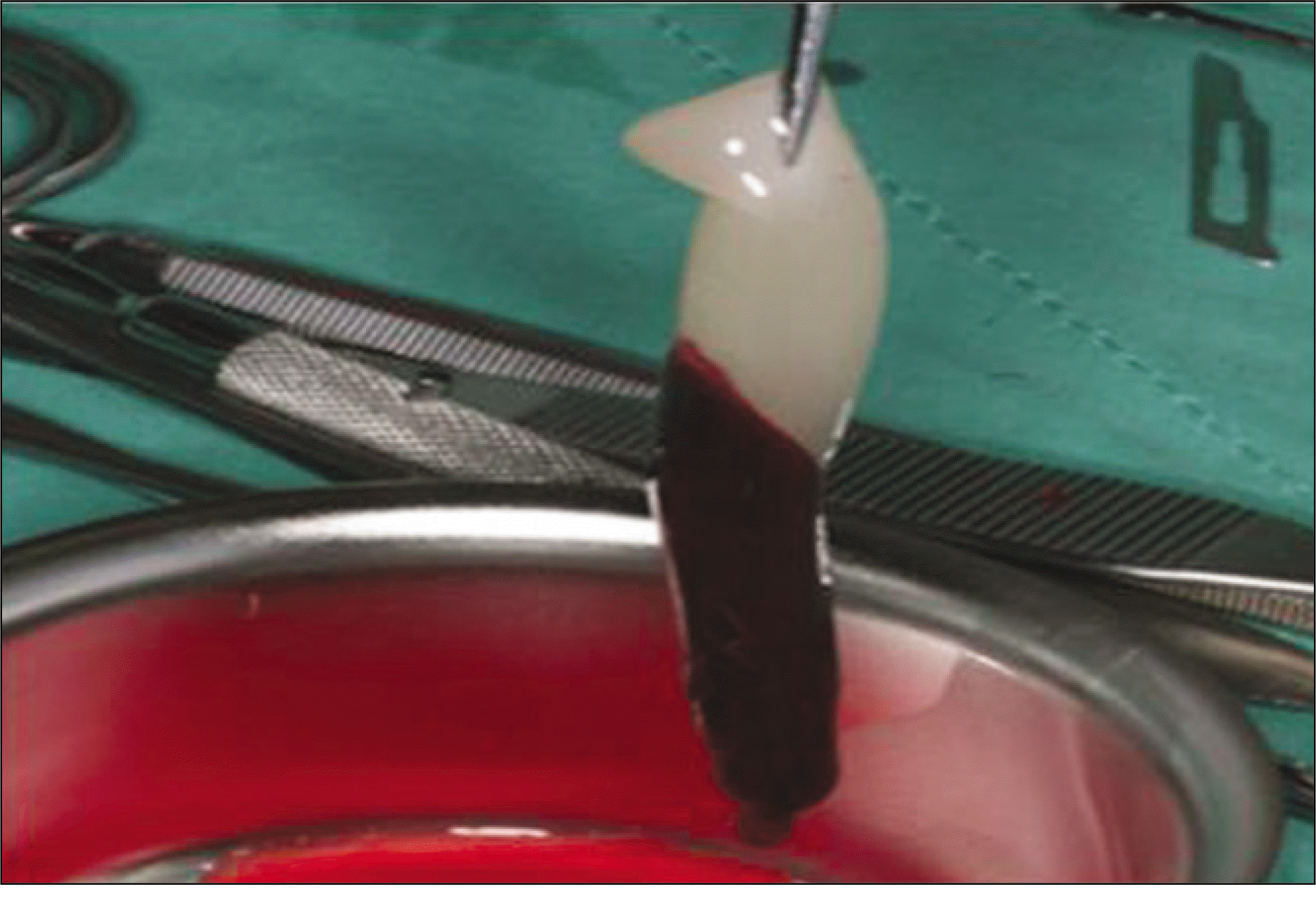 | Fig. 1.After centrifugation, the blood was separated in 3 layer. We used middle layer for grafting. |
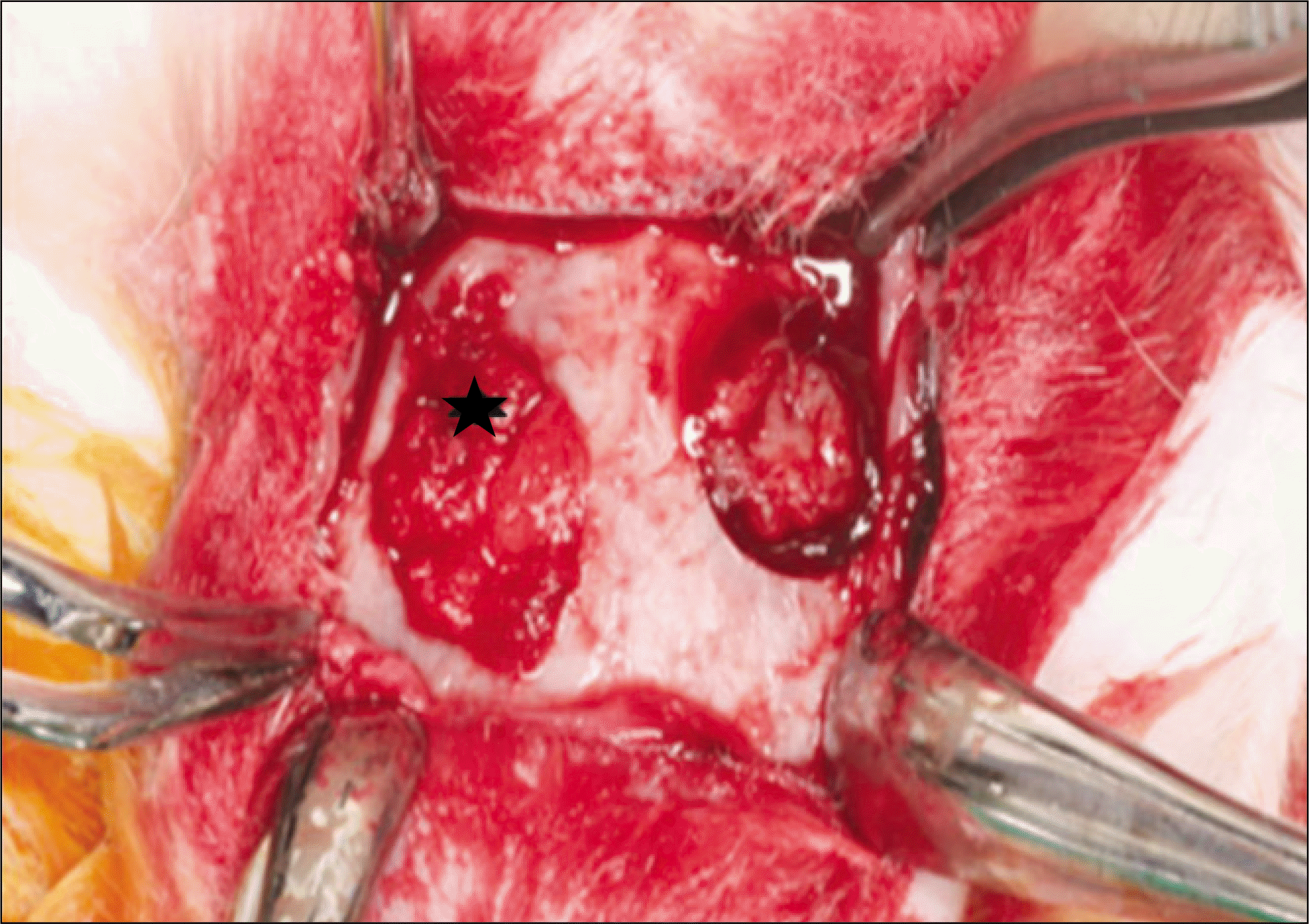 | Fig. 2.The silk protein mixed with PRF was grafted into the right parietal bone (experimental group: asterisk) and the left side (control group) was grafted only PRF. (PRF: platelet-rich fibrin) |
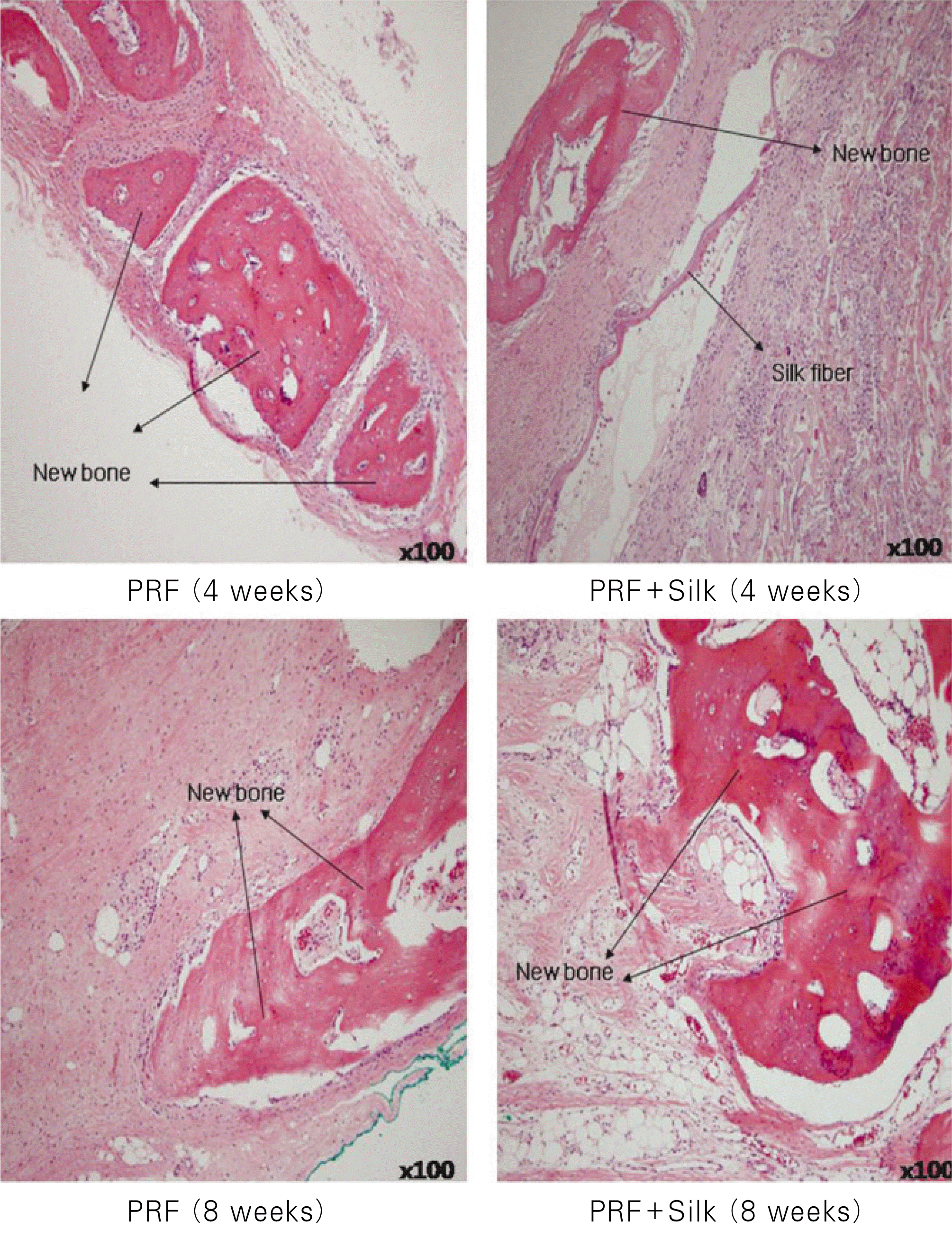 | Fig. 4.Histologic section (H&E staining, original magnification ×100). Osteoid formation both in the experimental group and in the control group at 4 weeks and 8 weeks. |
Table 1.
Microscopic computerized tomography analysis
| Sacrifice time | 4 weeks | 8 weeks | ||||
|---|---|---|---|---|---|---|
| Group | PRF | PRF+SILK | P value1 | PRF | PRF+SILK | P value1 |
| BMC (mg) | 365.61±25.10 | 361.92±25.96 | 0.19 | 419.52±20.89 | 414.32±30.53 | 0.34 |
| BMD (mg/cc) | 1217.63±86.38 | 1206.43±93.16 | 0.19 | 1394.11±70.99 | 1385.58±102.31 | 0.42 |
| TMC (mg) | 181.56±33.96 | 193.61±34.61 | 0.08 | 236.72±22.80 | 235.70±39.62 | 0.47 |
| TMD (mg/cc) | 2298.74±116.04 | 2376.26±135.72 | 0.04 | 2412.99±66.95 | 2425.66±86.76 | 0.15 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download