Abstract
Tissue engineered bone (TEB) can replace an autogenous bone graft requiring an secondary operation site as well as avoid complications like inflammation or infection from xenogenic or synthetic bone graft. Adult mesenchymal stem cells (MSC) for TEB are considered to have various ranges of differentiation capacity or multipotency by the donor site and age. This study examined the effect of age on proliferation capacity, differentiation capacity and bone morphogenetic protein-2 (BMP-2) responsiveness of human bone marrow stromal cells (hBMSC) according to the age. In addition, to evaluate the effect on enhancement for osteoblast differentiation, the hBMSC were treated with Trichostatin A (TSA) and 5-Azacitidine (5-AZC) which was HDAC inhibitors and methyltransferase inhibitors respectively affecting chromatin remodeling temporarily and reversibly. The young and old group of hBMSC obtained from the iliac crest from total 9 healthy patients, showed similar proliferation capacity. Cell surface markers such as CD34, CD45, CD90 and CD105 showed uniform expression regardless of age. However, the young group showed more prominent transdifferentiation capacity with adipogenic differentiation. The osteoblast differentiation capacity or BMP responsiveness was low and similar between young and old group. TSA and 5-AZC showed potential for enhancing the BMP effect on osteoblast differentiation by increasing the expression level of osteogenic master gene, such as DLX5, ALP. More study will be needed to determine the positive effect of the reversible function of HDAC inhibitors or methyltransferase inhibitors on enhancing the low osteoblast differentiation capacity of hBMSC.
Go to : 
References
1. Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999; 181:67–73.

2. Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, et al. Age-related intrinsic changes in human bone-marrowderived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008; 7:335–43.

3. Ma D, Ma Z, Zhang X, Wang W, Yang Z, Zhang M, et al. Effect of age and extrinsic microenvironment on the proliferation and osteogenic differentiation of rat dental pulp stem cells in vitro. J Endod. 2009; 35:1546–53.

4. Benetti R, Garcia-Cao M, Blasco MA. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nat Genet. 2007; 39:243–50.

5. Gonzalo S, Jaco I, Fraga MF, Chen T, Li E, Esteller M, et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol. 2006; 8:416–24.

6. Schroeder TM, Westendorf JJ. Histone deacetylase inhibitors promote osteoblast maturation. J Bone Miner Res. 2005; 20:2254–63.

7. de Boer J, Licht R, Bongers M, van der Klundert T, Arends R, van Blitterswijk C. Inhibition of histone acetylation as a tool in bone tissue engineering. Tissue Eng. 2006; 12:2927–37.
8. Jeon EJ, Lee KY, Choi NS, Lee MH, Kim HN, Jin YH, et al. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem. 2006; 281:16502–11.

9. Atkinson SP, Keith WN. Epigenetic control of cellular senescence in disease: opportunities for therapeutic intervention. Expert Rev Mol Med. 2007; 9:1–26.

10. Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of my-oblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci U S A. 1995; 92:9856–60.

11. Ogawa A, Ohba K, Uchida Y, Wada K, Yoshioka T, Muraki T. New adipogenic cell lines derived from C3H10T1/2. In Vitro Cell Dev Biol Anim. 1999; 35:307–10.

12. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–7.

13. Fang H, Yang X, Chen A, Luo Y. Effect of rhBMP-2 and osteogenic revulsants on proliferation and differentiation of bone marrow stromal cells in rats. J Huazhong Univ Sci Technolog Med Sci. 2007; 27:561–3.

14. Osyczka AM, Diefenderfer DL, Bhargave G, Leboy PS. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs. 2004; 176:109–19.

15. Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002; 71:36–44.

16. Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, et al. Donor age and cell passage affects differentiation potential of murine bone marrowderived stem cells. BMC Cell Biol. 2008; 9:60.

17. Mizuno D, Agata H, Furue H, Kimura A, Narita Y, Watanabe N, et al. Limited but heterogeneous osteogenic response of human bone marrow mesenchymal stem cells to bone morphogenetic protein-2 and serum. Growth Factors. 2010; 28:34–43.

Go to : 
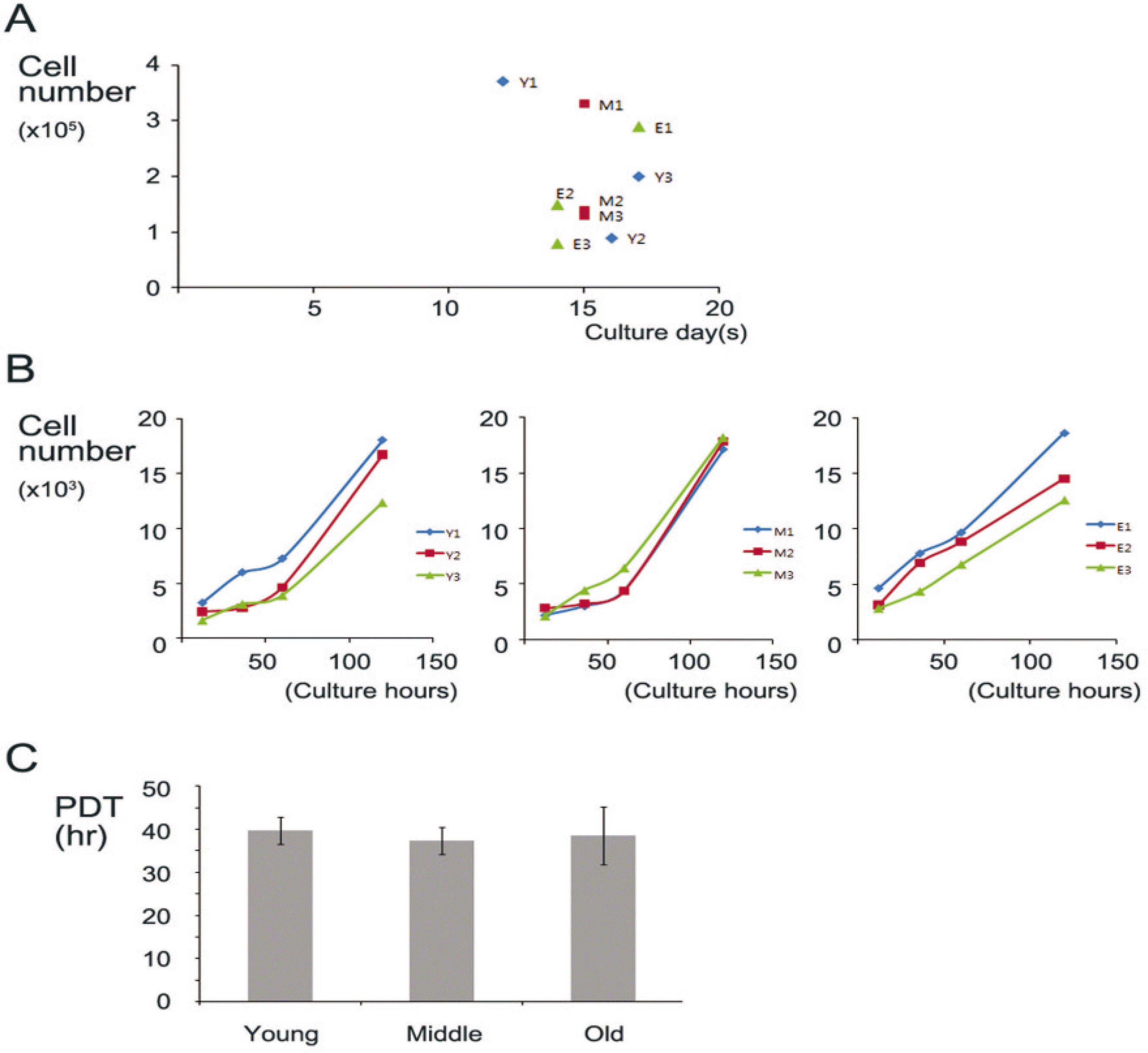 | Fig. 1.Proliferation assay of hBMSC. A. Total cell numbers of harvested hBMSC after P0 culture was manually counted by hemocytometer between culture day D13 and D17. B. CCK-8 assay was performed after 12, 36, 60, 120 hours culture with P3–5 cells of respective hBMSC. C. Population doubling time (PDT) was analyzed. (CCK: cell counting kit) |
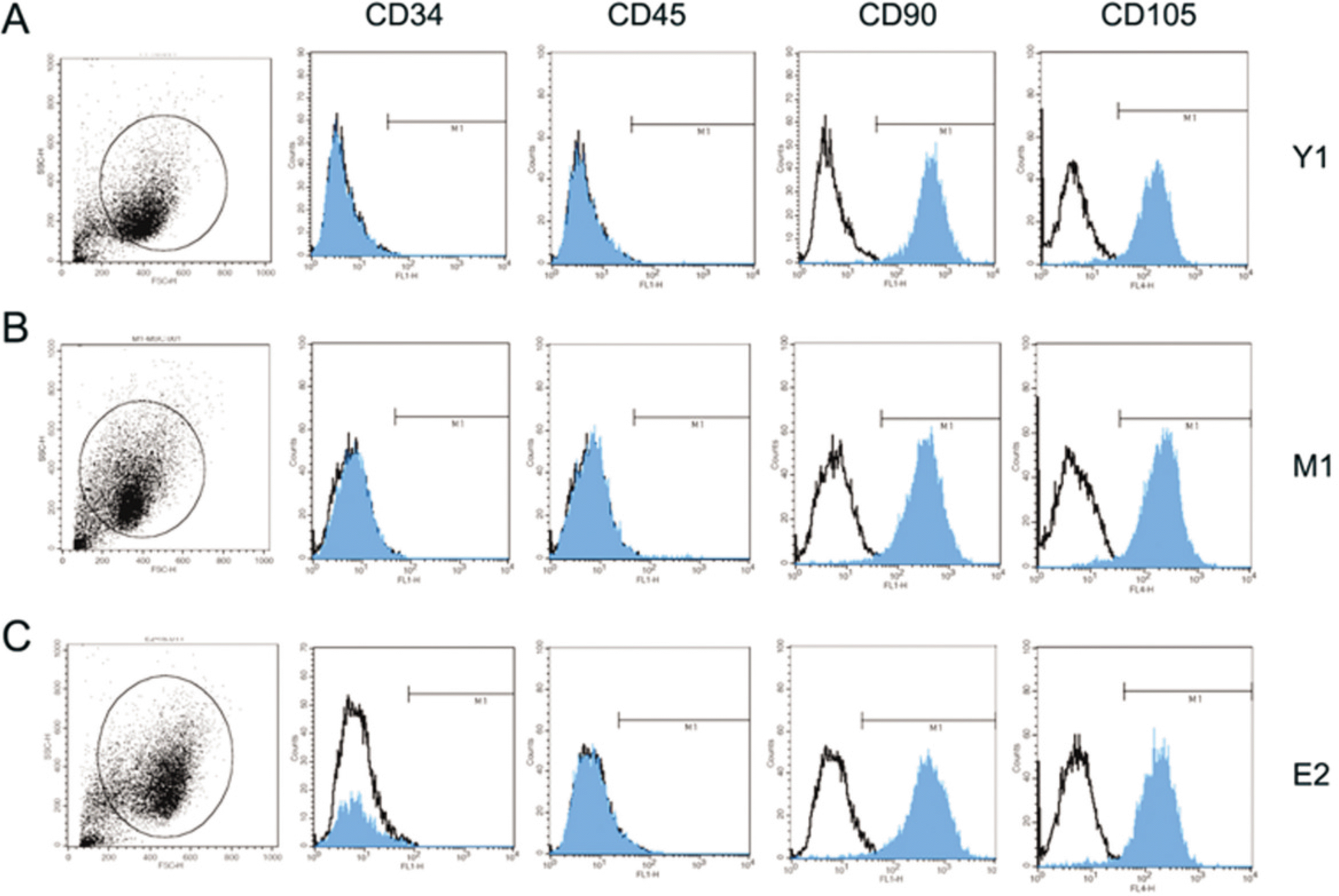 | Fig. 2.Surface marker expression of hBMSC between young and old aged group. FACS of the immunophenotypic surface profile for CD34, CD45, CD90 and CD105 from isolated hBMSC was analyzed. Black line empty histograms represent the fluorescence from negative-control cells incubated without antibody: blue colored histograms represent the counts of singals incubated with the relevant cell surface antibody. (FACS: fluorescence-activated cell sorting) |
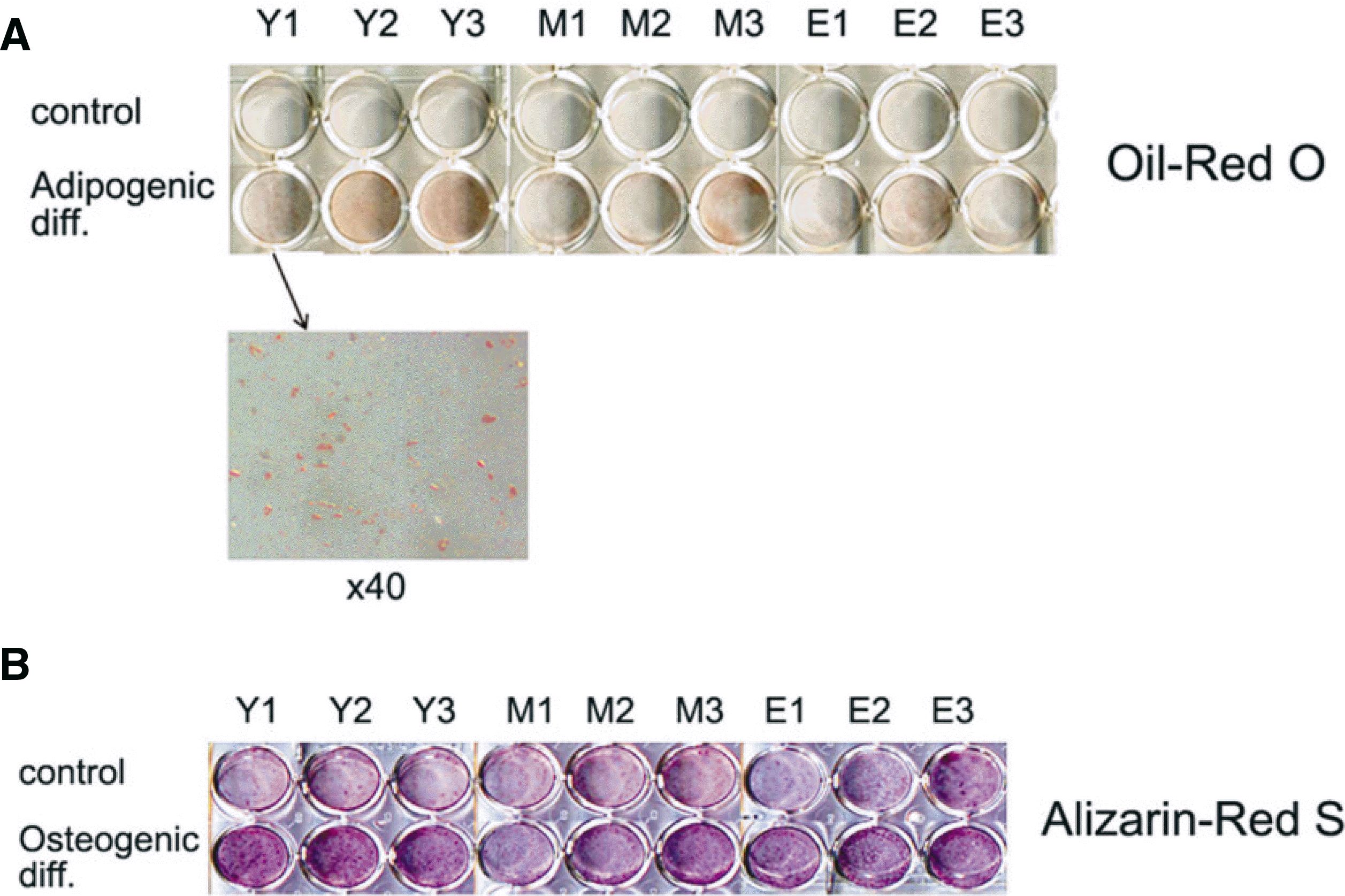 | Fig. 3.Multipotency of hBMSC. A. Oil-red O staining after adipogenic differentiation for 2 weeks in adipogenic medium. B. Alizarin-Red S staining after osteogenic differentiation for 2 weeks in osteogenic medium. |
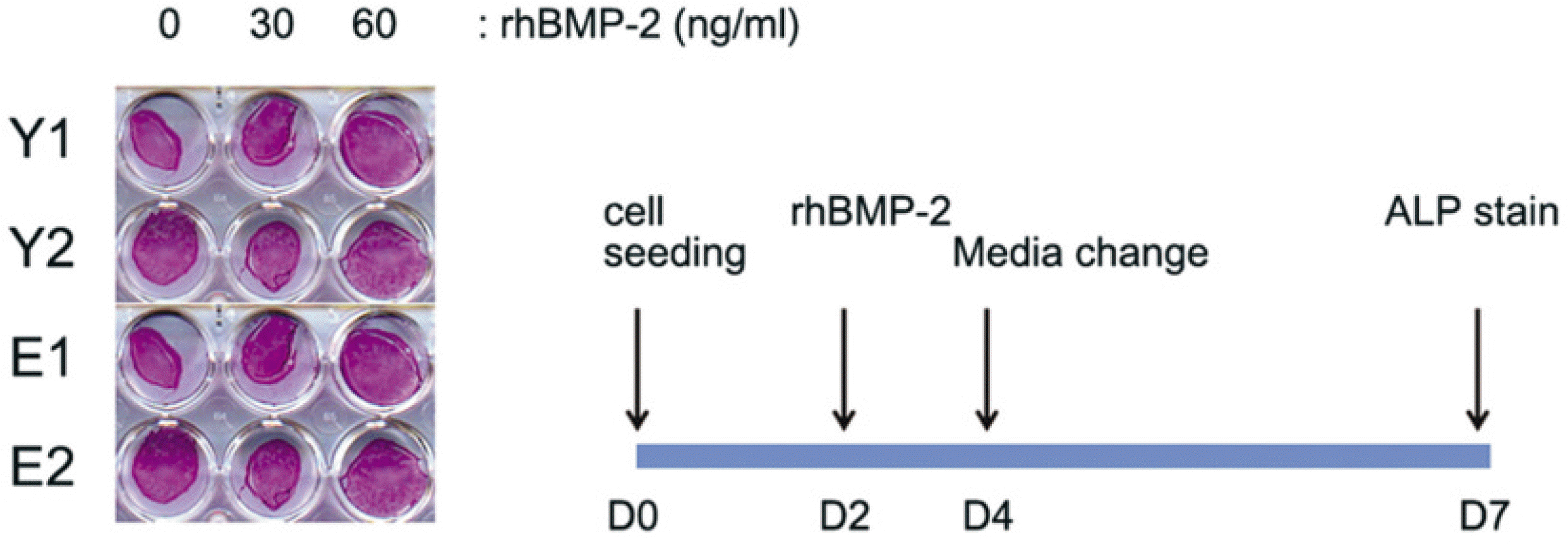 | Fig. 4.Low rhBMP-2 responsiveness of hBMSC. (rhBMP-2: recombinant human bone morphogenetic protein-2) |
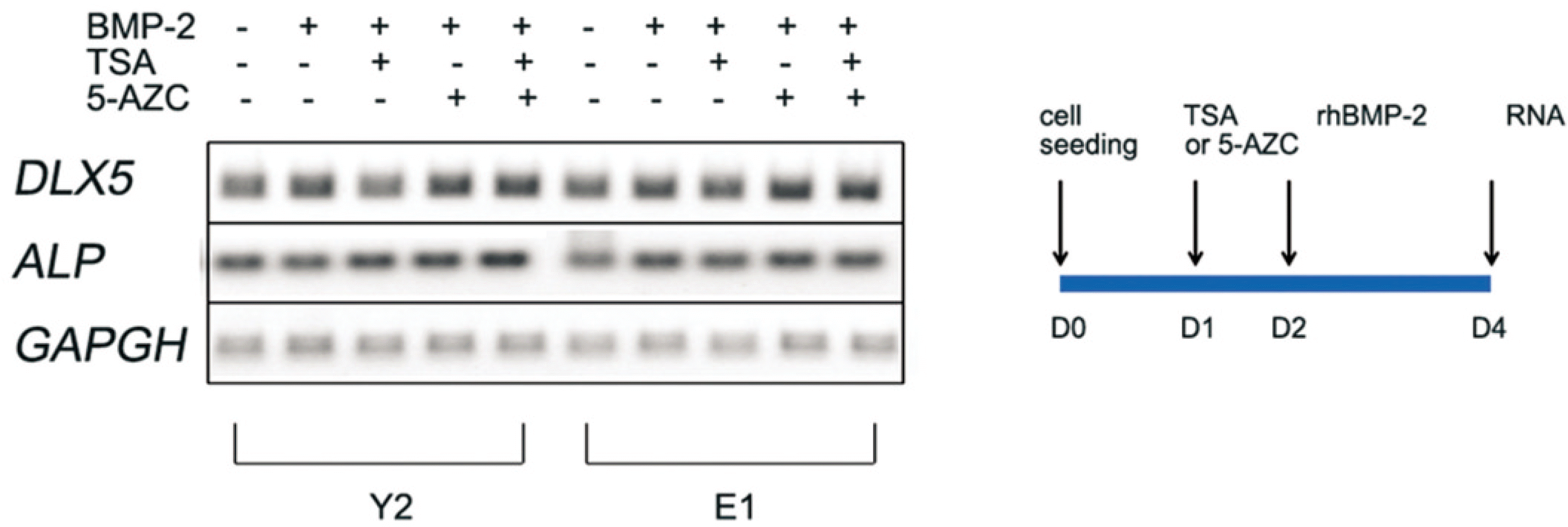 | Fig. 5.Osteogenic marker gene expression of hBMSC after TSA, 5-AZC pretreatment by semiquantitive RT-PCR. (TSA: trichostatin A, 5-AZC: 5-azacytidine, DLX5: distal-less homeobox 5, ALP: Alkaline phosphatase, GAPDH: glyceraldehyde-3-phosphate dehydrogenase) |
Table 1.
Characteristics of hBMSC donors from iliac crest
| Subject | Age/Sex |
|---|---|
| Y1 | 21/M |
| Y2 | 25/F |
| Y3 | 26/F |
| M1 | 39/F |
| M2 | 39/F |
| M3 | 33/F |
| E1 | 54/F |
| E2 | 61/F |
| E3 | 70/M |
Table 2.
Primer sets for RT-PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download