Abstract
Introduction
This study was to assess the effectiveness of new bone formation and regeneration by using a rhBMP-2 and β-TCP as a carrier in rabbits’ mandible.
Materials and Methods
The mandibles of 36 rabbits were exposed and cortical bone was penetrated for this study. The experimental subjects were divided into 3 groups each 12 rabbits; control group, experimental group 1, and experimental group 2. Control group had the defect itself without any treatment, in the experimental group 1, β-TCP only was grafted, and in the experimental group 2, rhBMP-2 soaked in β-TCP was grafted. The rabbits were sacrificed after 1, 2, 3, 4, 6, and 8weeks, and new bone formation area was examined and measured for bone quantitative and qualitative analysis with light, fluorescent and polarized microscopy.
Go to : 
References
1. Proussaefs P, Lozada J. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation: a human study. Int J Periodontics Restorative Dent. 2005; 25:351–63.
2. Springfield D. Aograft reconstructions. Orthop Clin North Am. 1996; 27:483–92.
3. Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989; 60:655–63.

4. Sculean A, Stavropoulos A, Windisch P, Keglevich T, Karring T, Gera I. Healing of human intrabony defects following regenerative periodontal therapy with a bovine derived xenograft and guided tissue regeneration. Clin Oral Investig. 2004; 8:70–4.

5. Hallman M, Nordin T. Sinus floor augmentation with bovine hydroxyapatite mixed with fibrin glue and later placement of non-submerged implants: a retrospective study in 50 patients. Int J Oral Maxillofac Implants. 2004; 19:222–7.
6. Proussaefs P, Lozada J, Rohrer MD. A clinical and histologic evaluation of a block onlay graft in conjunction with autogenous particulate and inorganic bovine mineral (Bio-Oss): a case report. Int J Periodontics Restorative Dent. 2002; 22:567–73.
7. Taba M Jr, Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthod Craniofac Res. 2005; 8:292–302.

8. Dunn CA, Jin Q, Taba M Jr, Franceschi RT, Bruce Rutherford R, Giannobile WV. BMP gene delivery for alveolar bone engineering at dental implants defects. Mol Ther. 2005; 11:294–9.
10. Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988; 242:1528–34.

11. Hou LT, Liu CM, Liu BY, Chang PC, Chen MH, Ho MH, et al. Tissue engineering bone formation in novel recombinant human bone morphogenic protein 2-atelocollagen composite scaffolds. J Periodontol. 2007; 78:335–43.

12. Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop. 1998; 346:26–37.

13. Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000; 21:393–411.

14. Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003; 55:1613–29.

15. Saito N, Taka K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials. 2003; 24:2287–93.

16. Aldinger G, Herr G, Ku ¨sswetter W, Reis HJ, Thielemann FW, Holz U. Bone morphogenetic protein: a review. Int Orthop. 1991; 15:169–77.

17. Moskow BS, Lubarr A. Histological assessment of human periodontal defect after durapatite ceramic implant. Report of a case. J Periodontol. 1983; 54:455–62.

18. Saffar JL, Colombier ML, Detienville R. Bone formation in tricalcium phosphate-filled periodontal intrabony lesions. Histological observations in humans. J Periodontol. 1990; 61:209–16.

19. Winn SR, Uludag H, Hollinger JO. Carrier systems for bone morphogenetic proteins. Clin Orthop. 1999; (367 Suppl):S95–106.

20. Kim HJ, Choi SM, Ku Y, Rhyu IC, Chung CP, Han SB, et al. The effect of rhBMP-2 on the osteoblastic differentiation of human periodontal ligament cells and gingival fibroblasts in vitro. J Periodontal Implant Sci. 2002; 32:389–402.

21. Granjeiro JM, Oliveira RC, Bustos-Valenzuela JC, Sogayar MC, Taga R. Bone morphogenetic proteins: from structure to clinical use. Braz J Med Biol Res. 2005; 38:1463–73.

22. Barboza EP, Duarte ME, Geola ′ s L, Sorensen RG, Riedel GE, Wikesjo ¨ UM. Ridge augmentation following implantation of recombinant human bone morphogenetic protein-2 in the dog. J Periodontol. 2000; 71:488–96.

23. Bessho K, Carnes DL, Cavin R, Chen HY, Ong JL. BMP stimulation of bone response adjacent to titanium implants in vivo. Clin Oral Implants Res. 1999; 10:212–8.

24. Ren WH, Yang LJ, Dong SZ. Induction of reparative dentin formation in dogs with combined recombinant human bone morphogenetic protein 2 and fibrin sealant. Chin J Dent Res. 1999; 2:21–4.
25. Jepsen S, Albers HK, Fleiner B, Tucker M, Rueger D. Recombinant human osteogenic protein-1 induces dentin formation: an experimental study in miniature swine. J Endod. 1997; 23:378–82.

26. Sigurdsson TJ, Fu E, Tatakis DN, Rohrer MD, Wikesjo ¨ UM. Bone morphogenetic protein-2 for peri-implant bone regeneration and osseointegration. Clin Oral Implants Res. 1997; 8:367–74.

27. Kato M, Toyoda H, Namikawa T, Hoshino M, Terai H, Miyamoto S, et al. Optimized use of a biodegradable polymer as a carrier material for the local delivery of recombinant human bone morphogenetic protein-2 (rhBMP-2). Biomaterials. 2006; 27:2035–41.

28. Tatakis DN, Koh A, Jin L, Wozney JM, Rohrer MD, Wikesjo ¨ UM. Peri-implant bone regeneration using recombinant human bone morphogenetic protein-2 in a canine model: a doseresponse study. J Periodontal Res. 2002; 37:93–100.

29. Jung RE, Weber FE, Thoma DS, Ehrbar M, Cochran DL, Ha ¨-mmerle CH. Bone morphogenetic protein-2 enhances bone formation when delivered by a synthetic matrix containing hydrox-yapatite/tricalciumphosphate. Clin Oral Implants Res. 2008; 19:188–95.

30. Yuan H, de Bruijn JD, Zhang X, van Blitterswijk CA, de Groot K. Use of an osteoinductive biomaterial as a bone morphogenetic protein carrier. J Mater Sci Mater Med. 2001; 12:761–6.
31. Liu Y, Huse RO, de Groot K, Buser D, Hunziker EB. Delivery mode and efficacy of BMP-2 in association with implants. J Dent Res. 2007; 86:84–9.

32. Bouxsein ML, Turek TJ, Blake CA, D'Augusta D, Li X, Stevens M, et al. Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2001; 83-A:1219–30.

33. Lee YM, Park YJ, Lee SJ, Ku Y, Han SB, Choi SM, et al. Tissue engineered bone formation using chitosan/tricalcium phosphate sponges. J Periodontol. 2000; 71:410–7.

34. Szabo ′G, Suba Z, Hraba ′ k K, Baraba ′ s J, Ne ′ meth Z. Autogenous bone versus beta-tricalcium phosphate graft alone for bilateral sinus elevations (2-and 3-dimensional computed tomographic, histologic, and histomorp hometric evaluations): preliminary results. Int J Oral Maxillofac Implants. 2001; 16:681–92.
35. Lu J, Descamps M, Dejou J, Koubi G, Hardouin P, Lemaitre J, et al. The biodegradation mechanism of calcium phosphate biomaterials in bone. J Biomed Mater Res. 2002; 63:408–12.

36. Jensen SS, Broggini N, Hj � rting-Hansen E, Schenk R, Buser D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-TCP. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res. 2006; 17:237–43.
Go to : 
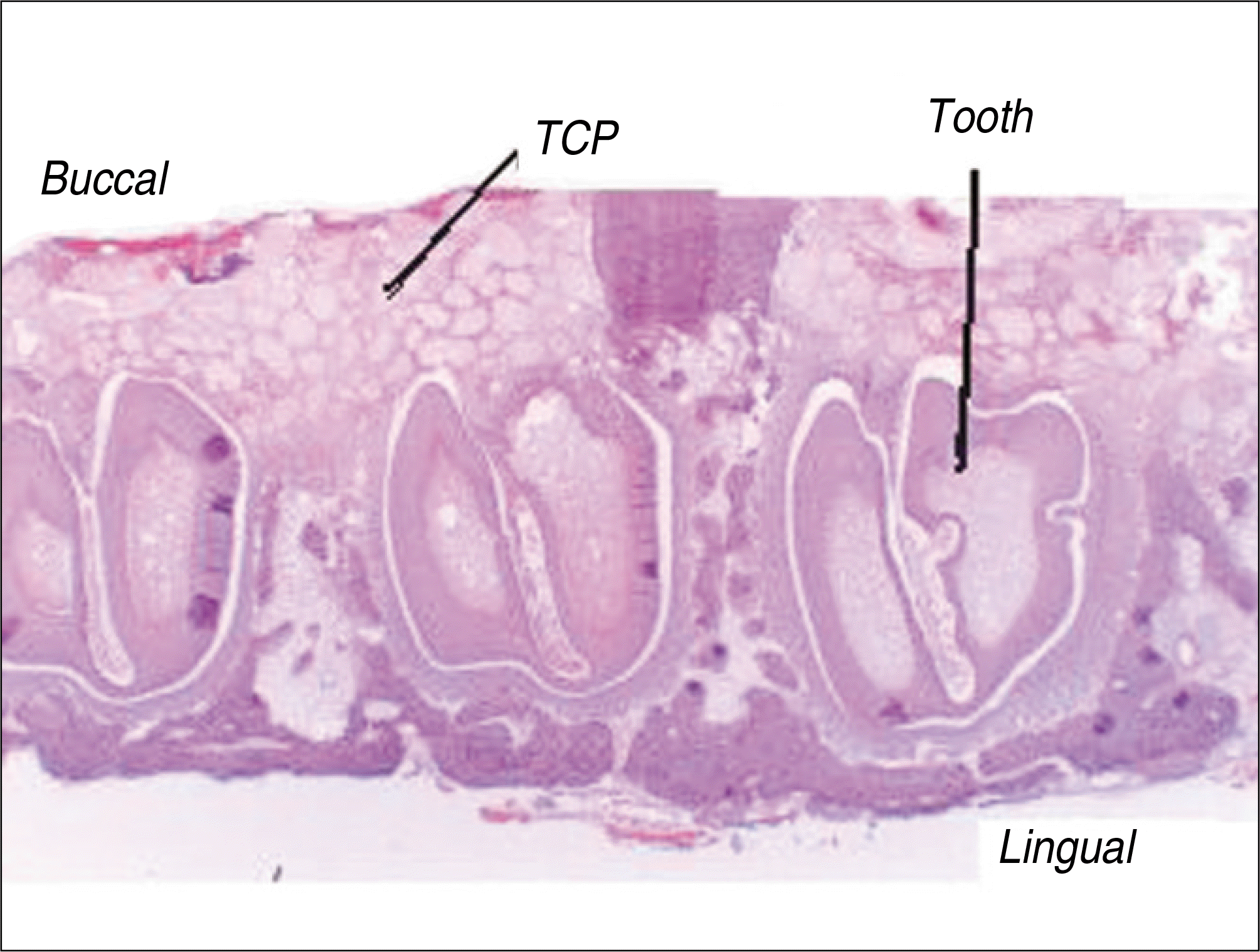 | Fig. 2.Photomicrographs of the histological section seen by light microscopy.(original magnification x10) (TCP: β-tricalcium phosphate) |
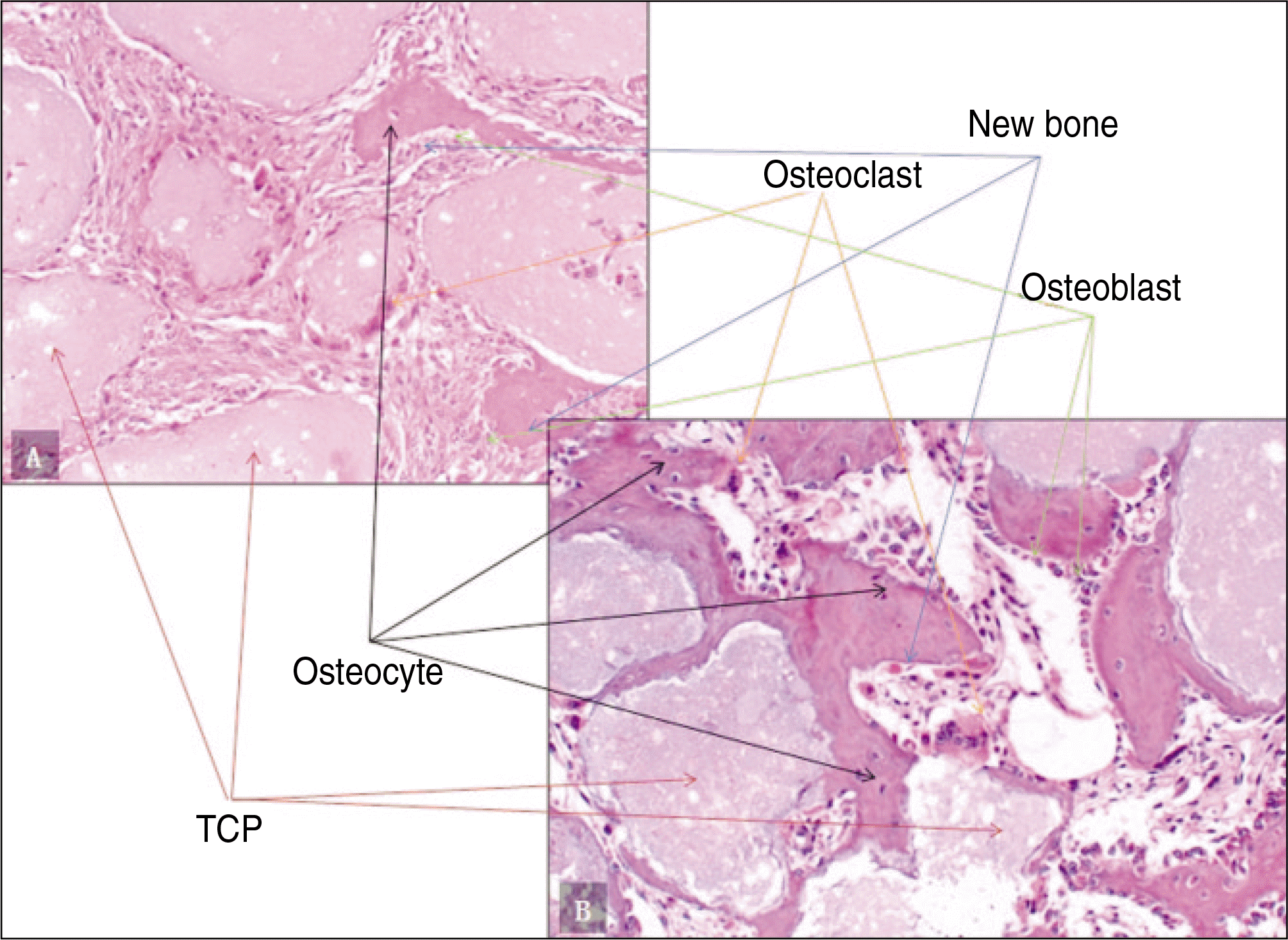 | Fig. 3.Photomicrographs of decalcified specimen.(H&E staining) A. Experimental group 1, 2 weeks.(original magnification x200) B. Experimental group 2, 2 weeks.(original magnification x200) (TCP: β-tricalcium phosphate) |
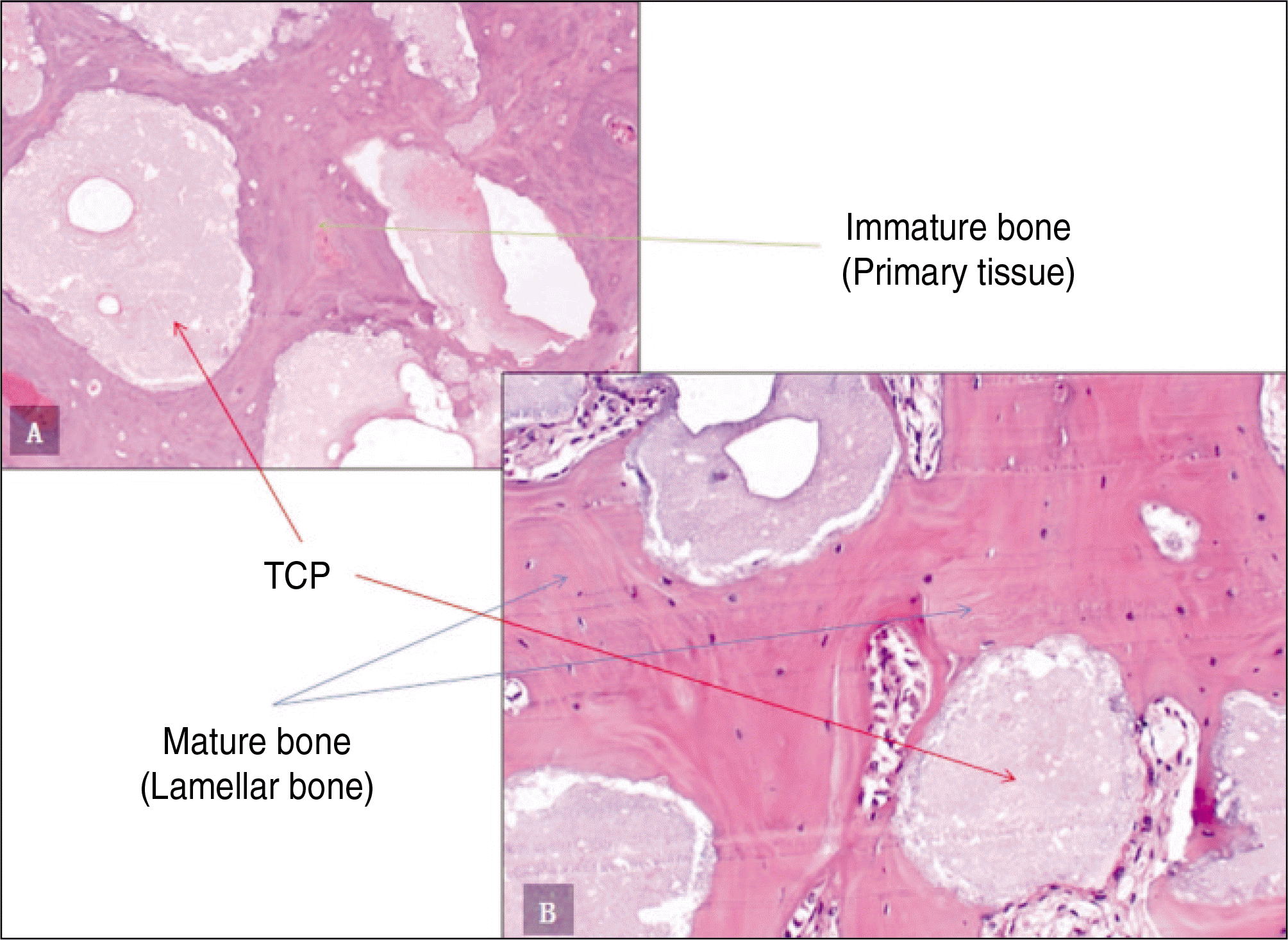 | Fig. 4.Photomicrographs of decalcified specimen.(H&E staining) A. Experimental group 1, 6 weeks.(original magnification x200) B. Experimental group 2, 6 weeks.(original magnification x200) (TCP: β-tricalcium phosphate) |
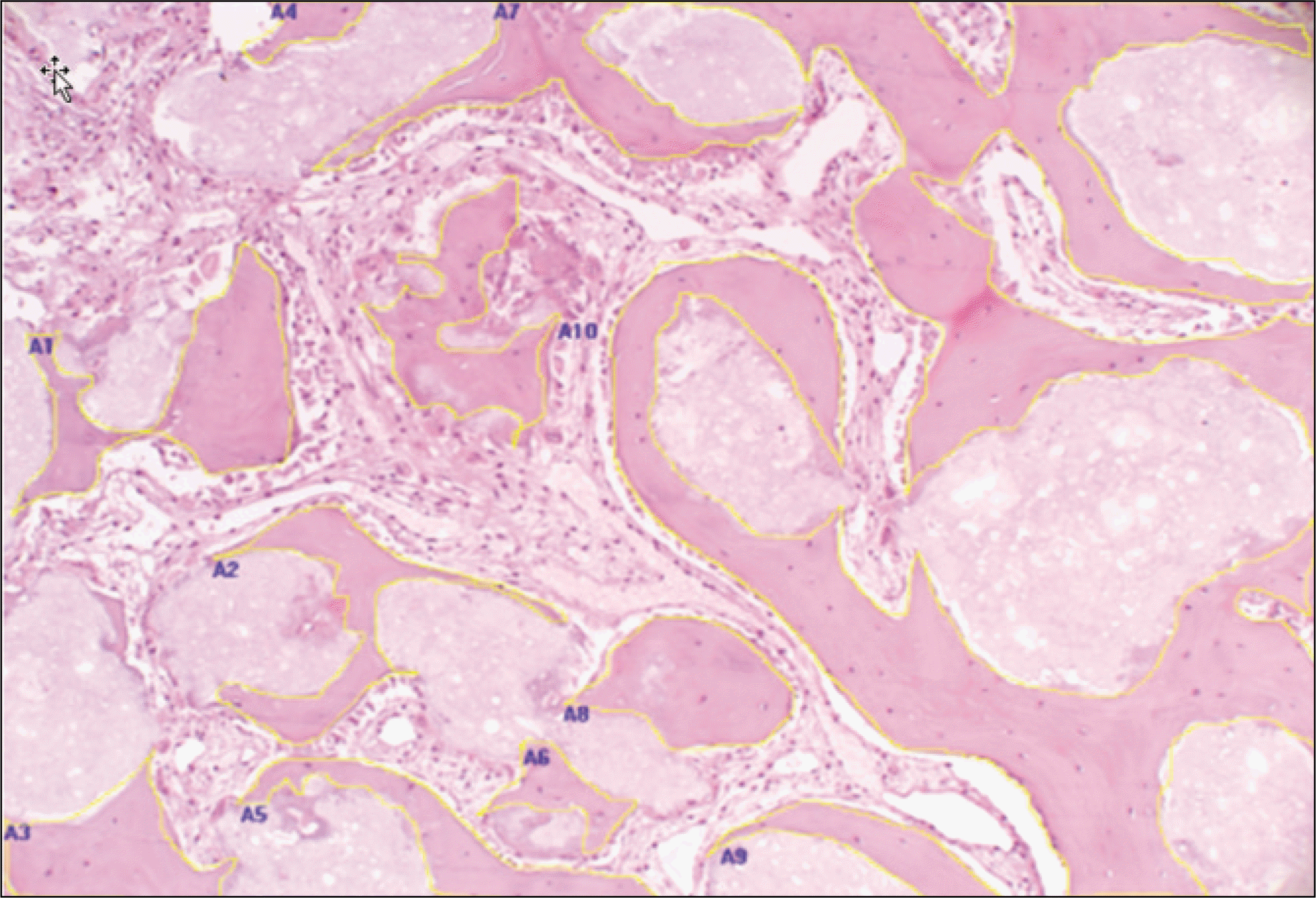 | Fig. 5.Quantitative analysis of bone formation at TCP/rhBMP-2 grafts. (H&E staining, original magnification x100, 4 weeks) Boundary of new bone formation were marked and calculated with Image Pro Plus 4.0.(A1-A10) (TCP: β-tricalcium phosphate, rhBMP-2: recombinant human bone morphogenetic protein-2) |
 | Fig. 6.Quantitative analysis of bone formation at control group (defect only), TCP grafts only and TCP/rhBMP-2 grafts. (H&E staining, original magnification x100) (TCP: β-tricalcium phosphate, rhBMP-2: recombinant human bone morphogenetic protein-2) |
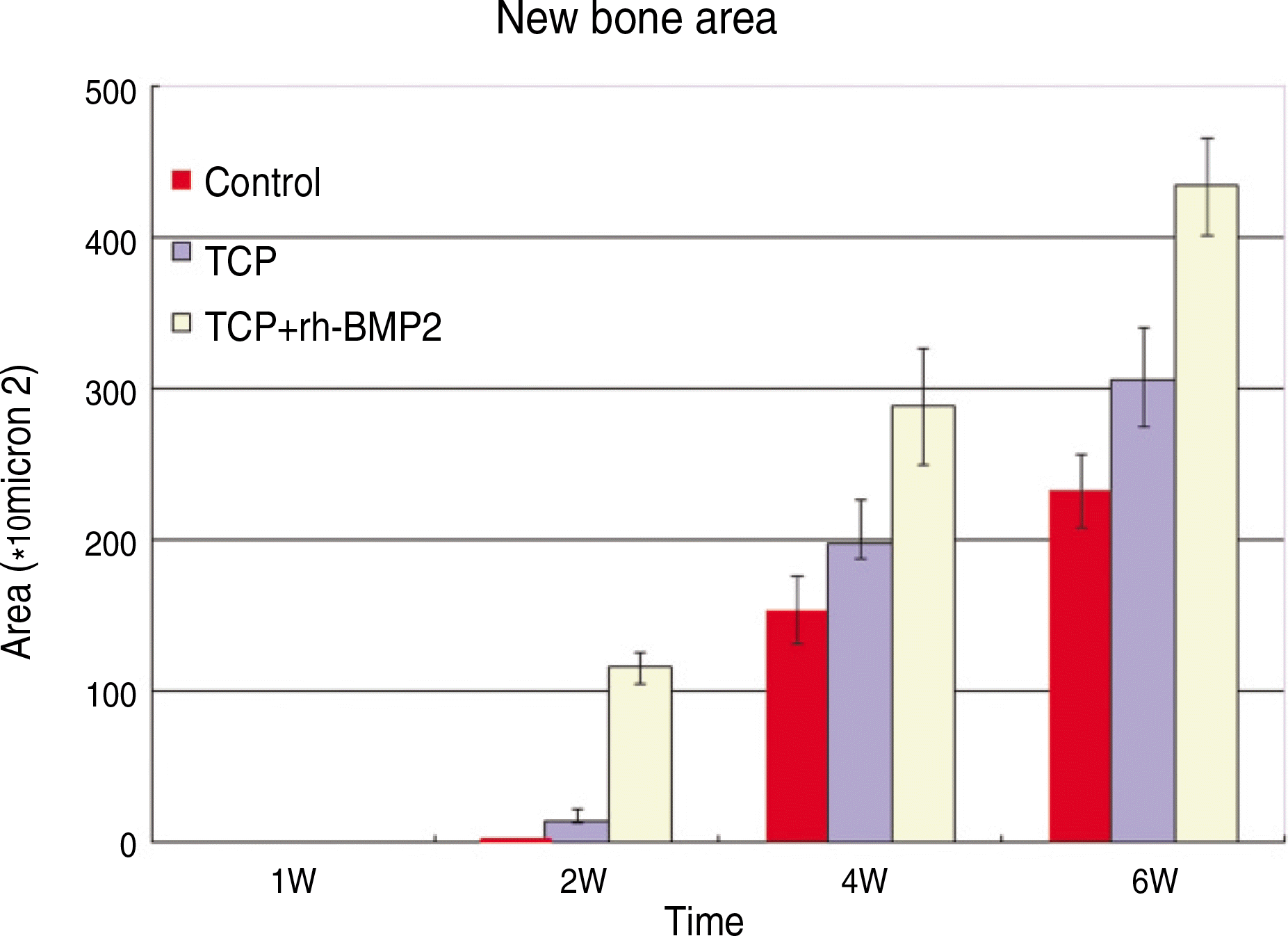 | Fig. 7.Quantitative analysis of bone formation at control group (defect only), β-TCP grafts only and β-TCP/rhBMP-2 grafts. (TCP: β-tricalcium phosphate, rhBMP-2: recombinant human bone morphogenetic protein-2) |
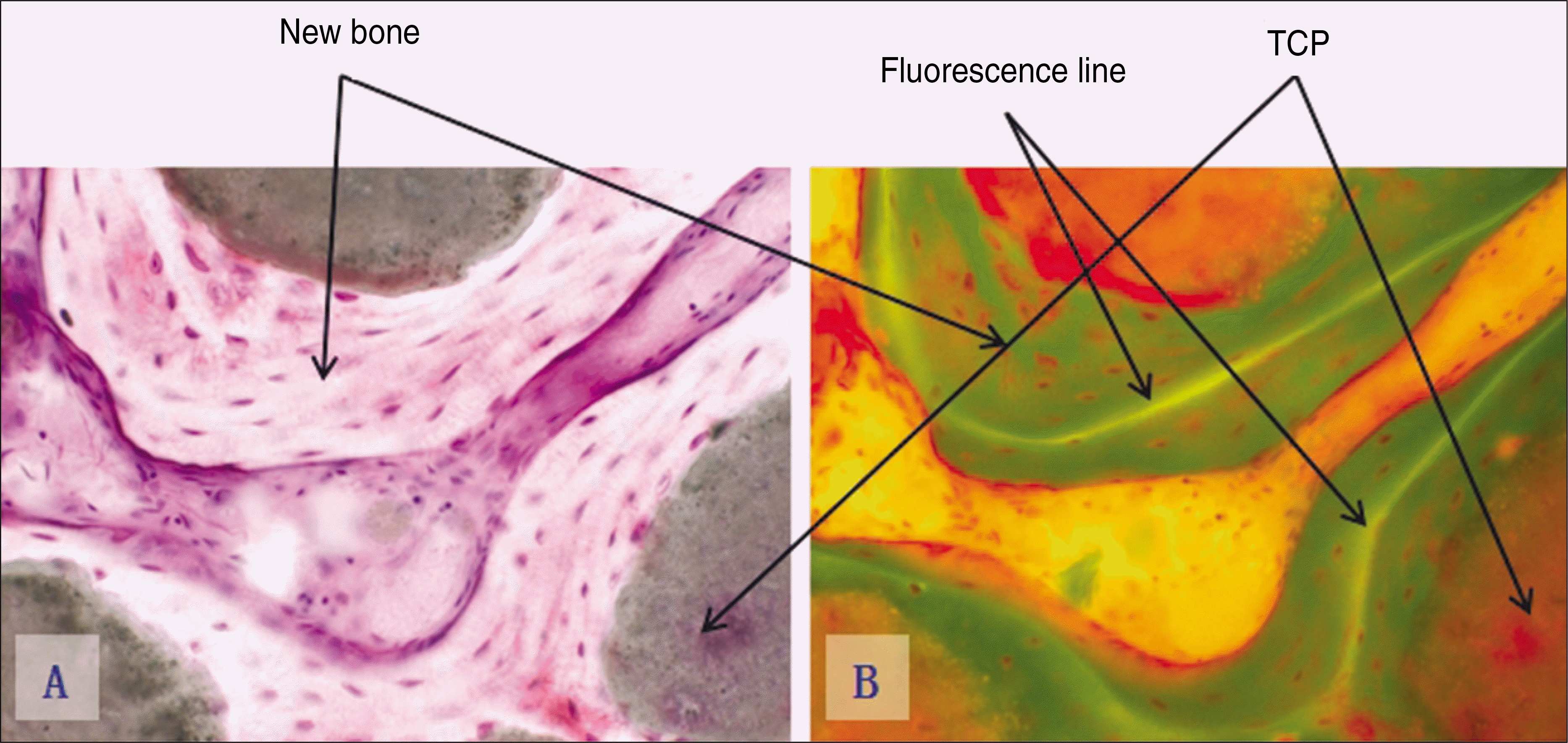 | Fig. 8.Photomicrographs of undecalcified specimen.(original magnification x100) A. Experimental group 2, 8 weeks. (Villanueva bone stain) B. Experimental group 2, 8 weeks.(fluorescence light) (TCP: β-tricalcium phosphate) |
 | Fig. 9.Comparison of Villanueva bone stain and fluorescence light microscopic photographs of undecalcified specimen at control group (defect only), TCP grafts only and TCP/rhBMP-2 grafts. (TCP: β-tricalcium phosphate, rhBMP-2: recombinant human bone morphogenetic protein-2) |
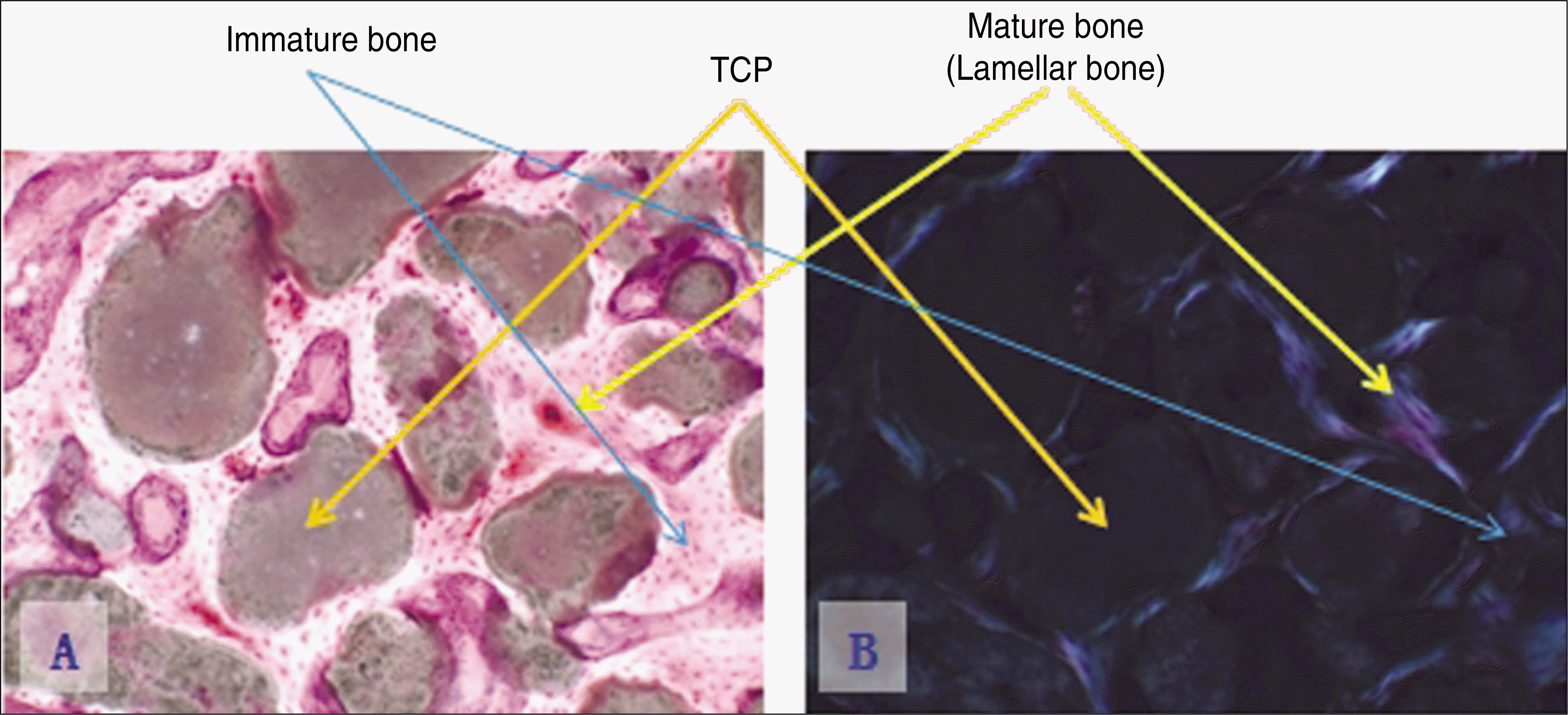 | Fig. 10.Photomicrographs of undecalcified specimen.(original magnification x100) A. Experimental group 2, 8 weeks.(Villanueva bone stain) B. Experimental group 2, 8 weeks.(polarizing light) (TCP: β-tricalcium phosphate) |
 | Fig. 11.Comparison of Villanueva bone stain and polarizing light microscopic photographs of undecalcified specimen at control group (defect only), TCP grafts only and TCP/rhBMP-2 grafts. (TCP: β-tricalcium phosphate, rhBMP-2: recombinant human bone morphogenetic protein-2) |
Table 1.
List of used graft materials
| Material | Manufacturer |
|---|---|
| β-TCP | Cowellmedi Co. Korea |
| rhBMP-2 | Cowellmedi Co. Korea |
Table 2.
Number of experimental animals in this study
| Experimental groups | Number of Rabbits | Site |
|---|---|---|
| Control group (defect only) | 12 | 24 |
| Experimental group 1 (β-TCP only) | 12 | 24 |
| Experimental group 2 (rhBMP-2/β-TCP) | 12 | 24 |
Table 3.
Quantitative analysis of bone formation area (Unit; %, 1/1.0208 mm2)
Table 4.
Comparative analysis of inter-grou up differences (Unit: times)
| Cont. vs Exp.1 | Cont. vs Exp.2 | Exp.1 vs Exp.2 | |
|---|---|---|---|
| 2 Wks | − | − | 8.72 |
| 4 Wks | 1.28 | 1.87 | 1.46 |
| 6 Wks | 1.31 | 1.87 | 1.42 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download