Abstract
Purpose
Ascorbic acid has been reported to have an adipogenic effect on 3T3-L1 preadipocytes, while evidence also suggests that ascorbic acid reduces body weight in humans. In this study, we tested the effects of ascorbic acid on adipogenesis and the balance of lipid accumulation in ovariectomized rats, in addition to long-term culture of differentiated 3T3-L1 adipocytes.
Materials and Methods
Murine 3T3-L1 fibroblasts and ovariectomized rats were treated with ascorbic acid at various time points. In vitro adipogenesis was analyzed by Oil Red O staining, and in vivo body fat was measured by a body composition analyzer using nuclear magnetic resonance.
Results
When ascorbic acid was applied during an early time point in 3T3-L1 preadipocyte differentiation and after bilateral ovariectomy (OVX) in rats, adipogenesis and fat mass gain significantly increased, respectively. However, lipid accumulation in well-differentiated 3T3-L1 adipocytes showed a significant reduction when ascorbic acid was applied after differentiation (10 days after induction). Also, oral ascorbic acid administration 4 weeks after OVX in rats significantly reduced both body weight and subcutaneous fat layer. In comparison to the results of ascorbic acid, which is a well-known cofactor for an enzyme of collagen synthesis, and the antioxidant ramalin, a potent antioxidant but not a cofactor, showed only a lipolytic effect in well-differentiated 3T3-L1 adipocytes, not an adipogenic effect.
The mortality rate of women with cessation of ovarian hormones, such as in menopause and with bilateral oophorectomy, increases due to cardiovascular diseases, such as coronary heart disease and stroke.12 As weight gain, a cardiovascular risk factor, is commonly followed by menopausal transition or bilateral oophorectomy, minimizing weight gain through diet and exercise is emphasized for reducing mortality.34
Ascorbic acid (vitamin C) is a cofactor in several enzymatic processes related to collagen, carnitine, and neurotransmitter synthesis, and its deficit results in scurvy.5 Collagen synthesis by ascorbic acid affects adipogenesis. Ascorbic acid increases the synthesis of type I and IV collagen and differential expression of type VI collagen in 3T3-L1 preadipocytes by enhancing differentiation of 3T3-L1 cells.67 Another well-known action of ascorbic acid is its antioxidant effect as an electron donor protecting against oxidative stress.8 Ascorbic acid has been reported to prevent osteoporosis and hip fracture through an antioxidant effect in postmenopausal women and ovariectomized mice.91011
Although the effect of ascorbic acid on weight gain in post-menopausal women and bilateral oophorectomized patients has not been reported yet, there are several papers suggesting that ascorbic acid as a lipolytic agent could be used in the treatment and prevention of obesity in human and animal models.12131415 The effect of ascorbic acid on weight gain should be clarified in post-menopause women and bilateral oophorectomy patients, as ascorbic acid has both an enhancing effect on adipogenesis of 3T3-L1 preadipocytes and a lipolytic effect. Therefore, we tested the effect of ascorbic acid on adipogenesis and lipolysis in the long-term culture of 3T3-L1 cells and ovariectomized rat models. In this study, we showed that application of ascorbic acid during the early period of 3T3-L1 cell differentiation and after ovariectomy (OVX) enhanced adipogenesis and body fat mass, respectively, while applying ascorbic acid during the late period decreased lipid accumulation and body fat mass.
Mouse embryo fibroblasts cells (3T3-L1 cells) were purchased from Korean Cell Line Bank. The cells were cultured in Dulbecco's Modified Eagles Medium (DMEM) supplemented with 10% bovine calf serum (BCS) for maintenance and cultured in DMEM with 10% fetal bovine serum (FBS) for chemical adipogenic induction. In order to produce mature adipocytes, 6.7×104 cells of 3T3-L1 were seeded on six-well plates with DMEM-BCS and cultured for 3 days to reach 100% confluence. The day on which cells reached confluency was referred to as Day -2, and they were treated with ascorbic acid (50 µg/mL) and/or ramalin (10 µg/mL) from this day on. After being grown for 2 more days in BCS media (Day 0), the cells were induced to be differentiated to adipocytes for 2 days with DMEM-FBS containing 1 µM dexamethasone and 250 µM 3-isobutyl 1-methyl xanthine. On Day 2, the cells were refreshed with DMEM-FBS containing insulin (10 µg/mL), and the media was replaced with DMEM-FBS every other day. In this study, treatment with ascorbic acid and ramalin was performed during two separate periods, early induction period of Day -2, Day 0, and Day 2, the late period of Day 10, Day 12, and Day 14, or during both periods. Induction chemicals and ascorbic acid were purchased from Sigma, and ramalin was generously gifted from Dr. Joung Han Yim at Korea Polar Research Institute.
To determine the degree of lipid accumulation, the 3T3-L1 cells were stained with Oil Red O (ORO, Sigma, St. Louis, MO, USA) on the 14th day after the induction of differentiation. The cells were fixed with 4% paraformaldehyde overnight and washed with 60% isopropanol. After drying the cells, 0.21% ORO in 60% isopropanol was applied to the cells for 10 min followed by washing four times with distilled water. Stained ORO was extracted with 100% isopropanol and absorbance was measured at 520 nm. Pictures were taken before extraction.
On Day 14, after a PBS wash, the 3T3-L1 cells were extracted with ×5 Laemmli buffer and 5% β-mercaptoethanol and boiled for 10 min. The samples were separated on sodium dodecyl sulfate (SDS)–polyacrylamide gels and transferred to nitrocellulose membranes for 1 hr in transfer buffer (25 mM Tris, 192 mM glycine, 0.1% SDS and 20% methanol, pH 8.3). Non-specific binding sites on the membranes were blocked in 5% non-fat dry milk for 90 min at room temperature, and the membrane was blotted with primary antibody at 4℃ overnight and secondary antibody for 90 min at room temperature. Blots were visualized using the chemiluminescence kit (Santa Cruz, Dallas, TX, USA). Primary antibody for collagen was a rabbit polyclonal antibody LF68 (a generous gift from Dr. Larry Fisher, NIDCR, NIH, Bethesda, MD, USA) against carboxy-telopeptide of α1(I) collagen and rabbit polyclonal antibodies against α1(VI) collagen (Santa Cruz). Rabbit polyclonal antibody to CCAAT/enhancer binding protein (CEBP) α and mouse monoclonal antibody to peroxisome proliferator-activated receptor (PPAR) γ were purchased from Santa Cruz for adipocyte differentiation regulating protein. Mouse monoclonal antibody to β-actin (Santa Cruz) was used for loading control.
3T3-L1 cells were grown on glass coverslips to Day 14 at 37℃ in six-well tissue culture plates. After the removal of media, the cells were washed in PBS for 5 min and then fixed in 4% paraformaldehyde overnight. They were then washed twice with PBS for 15 min and blocked with blocking solution (2% normal rabbit serum in PBS) for 30 min, followed by the addition of rabbit polyclonal antibody against collagen type I (Rockland Immunochemicals Inc., Limerick, PA, USA) in blocking solution (1:200) and incubation for 2 h at room temperature. After rinsing the cells twice with PBS for 5 min at room temperature, the cells were treated with biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories, Burlington, CA, USA) in PBS (1:400) for 30 min at room temperature. Coverslips were washed three times with PBS for 10 min, and antigen-antibody complexes were detected using an avidin-biotin complex detection system (Vectastain ABC Kit, Vector Laboratories). Coverslips were stained with DAB Substrate kit (Vector Laboratories), rinsed in water, briefly counter-stained with hematoxylin, and washed again in water. After mounted on glass slides, coverslips were examined with an Olympus BX51 (Olympus Corporation, Tokyo, Japan) microscope. Pictures were captured and controlled in Olympus DP72 and DP2-BSW.
Female Sprague Dawley rats (8 weeks old) weighing 200 g were purchased from Orient Bio Inc. (Seongnam, Korea). They were housed in a controlled environment (25±2℃, 50±10% humidity) and were provided with chow and water ad libitum. Four-fifths of the rats were ovariectomized bilaterally through midline abdominal incisions after deep anesthesia (50 mg/kg of ketamine and 250 mg/kg of xylazine), and the remaining animals were subjected to sham operations. The animal experiment was approved by the Institutional Animal Care and Use Committee of Korea University (Korea-2016-0025). The rats were then divided into the following five treatment groups: sham-operated control rats receiving vehicle (n=8), ovariectomized rats receiving vehicle (n=8), ovariectomized rats receiving ascorbic acid at Weeks 5 and 6 (late period) after surgery (n=8), ovariectomized rats receiving ascorbic acid at Week 1 and 2 (early period) after surgery (n=8), and ovariectomized rats receiving ascorbic acid at the early and late periods after surgery (n=8). Ascorbic acid was dissolved in water and given daily by oral injection at a concentration of 1 g/kg. Water was used as the vehicle. Right before and six weeks after OVX, anesthetized by ketamine (Yuhan Co., Seoul, Korea, 50 mg/kg) and xylazine hydrochloride (Sigma Co., 250 mg/kg), the rats were weighed, and their body fat was measured by a body composition analyzer of a nuclear magnetic resonance system (Minispec LF90, Bruker Co., Billerica, MA, USA). Hypodermis in the flank for subcutaneous adipose tissue and visceral fat mass of greater omentum were obtained and fixed with formalin for histological investigation.
After formalin fixed tissues were embedded in paraffin, 4-µm thick sections were stained with hematoxylin and eosin (H&E). After mounted them on slides using mounting medium (Clarion mounting medium, Biomeda), the slides were examined with an Olympus BX51 microscope. Pictures were captured and controlled in Olympus DP72 and DP2-BSW.
The data were expressed as the mean±standard error of the mean in bar graphs. Two-way analysis of variance was used to compare the experimental and control groups, and Tukey test was used to compare the significance between two groups. Statistical significance was determined as p-value less than 0.05 (*) or 0.01 (**).
In our previous paper,6 3T3-L1 preadipocytes were cultured for 8 days after adipogenic induction, and ascorbic acid increased the accumulation of triglycerides in these cells, especially during the early period of adipogenesis. In this study, to show the effect of ascorbic acid on long-term culture, 3T3-L1 cells were maintained for up to 14 days for differentiation into mature adipocytes. The accumulation of triglycerides in cells sharply increased up to 8 days after adipogenic induction. Then, it showed a plateau without ascorbic acid, but steadily increased with ascorbic acid treatment during the early period (Fig. 1A). However, adding ascorbic acid during the late period of adipogenesis, with or without ascorbic acid treatment during the early period, interestingly, reduced the ORO staining of triglycerides (p<0.05) (Fig. 1B). Although specific markers for lipolysis were not examined, this reduction of ascorbic acid during the late period could be regarded as lipolysis of adipocytes, because most 3T3-L1 preadipocytes had differentiated into mature adipocytes by 8 days after adipogenic induction.
In our previous study, we also showed that the differential expression of type I and VI collagen enhanced the accumulation of lipid during adipogenesis of 3T3-L1 preadipocytes.6 Thus, to investigate the effect of ascorbic acid as a cofactor of collagen synthesis on this reduction, type I collagen was detected in cell extracts and fixed cells. In the immunoblotting of the cell extracts, the expression of mature α1(I) collagen was increased by adding ascorbic acid during the late period, whereas procollagen decreased. However, the α1(VI) collagen and adipogenic markers, CEBPα and PPARγ, were similarly expressed, indicating that ascorbic acid could not affect the expression of well-known adipogenic agents during the late period (Fig. 1C). These immunoblotting results suggest that the reduction of lipid in adipocytes may not be induced by the collagen synthesis activity of ascorbic acid on early adipogenesis period.
In immunocytochemical examination, the staining pattern of type I collagen in formalin-fixed cells was not different between the two groups, with and without ascorbic acid treatment during the late period, while the number of type I collagen unstained cells (arrow) increased in the group that was treated with ascorbic acid during the late period (Fig. 1D). This suggests that a weakened cell barrier enabled the loss of lipid droplets of adipocytes and explains the reduced triglyceride staining in Fig. 1B.
To investigate the anti-oxidant activity, another action of ascorbic acid, of lipid reduction during adipogenesis, we applied ramalin, a stronger antioxidant than ascorbic acid, to adipogenesis of 3T3-L1 during the early and late periods. When ramalin was added during the early period, lipid accumulation did not increase like ascorbic acid, which is not strange, as the collagen synthesis activity of ramalin has not been reported. However, when ramalin was added during the late period, lipid accumulation was significantly inhibited (p<0.01), which was much more than ascorbic acid (Fig. 2A and B, 50% reduction for ramalin, and compared to Fig. 1B, 11% reduction for ASC), indicating that antioxidant activity is a major factor for reduced adipogenesis or lipolysis. This strong inhibition on lipid accumulation of ramalin during the late period was also shown when ascorbic acid was added during the early period of adipogenesis (Fig. 2A and B). Interestingly, even when ramalin was treated during the early period with ascorbic acid, ramalin significantly decreased the strong adipogenesis induced by ascorbic acid, which suggests that the antioxidant activity of ramalin might weaken the adipogenesis induced by ascorbic acid through collagen synthesis.
To apply this effect of ascorbic acid on adipogenesis in in vivo experiment, ascorbic acid was injected orally into the ovariectomized rat during the early or late period after OVX. At 6 weeks after the removal of both ovaries, the ovariectomized rat gained more body weight and body fat than sham control, confirming that the cessation of ovarian hormones in females induces weight gain through fat accumulation (Fig. 3A and B). Interestingly, when ascorbic acid was injected during 2 weeks of the late period after OVX, total body weight was significantly reduced, compared to other ovariectomized rat groups (Fig. 3A). For the body fat gain among ovariectomized rats, the increase of fat upon late injection of ascorbic acid was much less than that for early injections or early-late injections of ascorbic acid and similar to the OVX control (Fig. 3B).
Histologically, the thickness of subcutaneous fat layer increased at 6 weeks after OVX (sham vs. OVX none) (Fig. 4). Like the in vitro result, injection of ascorbic acid during the early period after surgery expanded the subcutaneous fat layer, compared to sham control or OVX control (OVX early vs. sham or OVX none) (Fig. 4). Intriguingly, the thickness of the subcutaneous fat layer was reduced to the extent of sham control when ascorbic acid was injected only during the late period after surgery (OVX late vs. sham) (Fig. 4). However, even though ascorbic acid was injected during the late period, subcutaneous fat layer did not decrease when ascorbic acid was injected during the early period (OVX early vs. OVX early-late) (Fig. 4). The H&E staining of visceral adipocytes in greater omentum in sham control showed diverse sizes of adipocytes, whereas those in OVX presented enlarged adipocytes (OVX vs. sham) (Fig. 4).
Ascorbic acid is a cofactor in the enzymatic process of collagen synthesis and enhances the adipogenesis of 3T3-L1 cells with type IV and VI collagen as structural proteins of cells.67 In the culture of human skin, ascorbic acid also increased the formation of multiple lipid lamellar structures.16 In this study, ascorbic acid showed the significant increase of adipogenesis and lipid accumulation of 3T3-L1 cells, which was maintained for 14 days when it was applied during the induction period. These effects of ascorbic acid in in vitro experiments were also observed here in the animal obesity model induced by bilateral OVX. The ascorbic acid that was orally administered right after OVX for 2 weeks tended to increase body fat mass, body fat ratio, subcutaneous fat (depth), and visceral fat (size of adipocytes), although the differences were statistically insignificant.
Meanwhile, lipid accumulation of adipocytes is another process, the balance of lipogenesis and lipolysis. After OVX, rats and mice gained body weight, showing an increase in both total adipose tissue mass and lean body mass associated with hyperphagia.17 Although the mechanism was not clear, the lack of estradiol did not inhibit the decrease of eating in the estrus cycle and induced a tonic increase of eating probably due to the decrease of satiating hormones of CCK, GLP-1, and glucagon.17 In this study, body weight rapidly increased until 4 weeks after OVX and then maintained, as has been shown in previous papers.1819 Interestingly, the administration of ascorbic acid in this maintenance period after OVX significantly reduced body weight gain and subcutaneous fat layer, while the administration of ascorbic acid right after OVX resulted in a slight increase therein. Similar to the in vivo experiment, lipid accumulation was significantly reduced by ascorbic acid, which was treated to well-differentiated 3T3-L1 adipocytes. This reduction of lipid accumulation might result from the antioxidant effect of ascorbic acid rather than the action as a cofactor and be reproduced with another antioxidant substance, ramalin. Ramalin has been recently discovered to be a potent non-toxic antioxidant from Antarctic lichen and is 1.2 times more potent than ascorbic acid in scavenging superoxide radicals.20 Ramalin has been reported to reduce lipid accumulation in 3T3-L1 preadipocyte culture.2021 In this study, ramalin significantly reduced lipid accumulation in well-differentiated 3T3-L1 adipocytes, both with and without ascorbic acid treatment. In comparison, as seen in Fig. 1A and 2B, the addition of ramalin during the late period decreased lipid accumulation more than that of ascorbic acid in 3T3-L1 cells. This suggested that, if a more potent antioxidant was used, a higher level of body fat reduction would occur. However, contrary to ascorbic acid, ramalin is unable to facilitate the synthesis of collagen ability, and for this reason, ramalin did not increase adipogenesis when administered during the early period (Fig. 2B). Therefore, we suggest that ascorbic acid would have two different functions, a cofactor of collagen synthesis for adipogenesis and an antioxidant for lipolysis.
While it is evident that ascorbic acid has a potent antioxidant function, there is a debate concerning the effect of ascorbic acid on body weight reduction clinically. It has been reported that ascorbic acid supplementation reduced body weight, insulin resistance and atherosclerosis1222 and that there was an inverse relationship between serum ascorbic acid and body weight and cardiovascular diseases.2324 However, cohort study about the relationship between body mass index and dietary intake of ascorbic acid has shown that ascorbic acid might be weakly related to a reduction in body weight and waist circumference only in obese people who are genetically predisposed to a high waist-hip-ratio.25
It is clinically important to clarify whether ascorbic acid plays a beneficial or harmful role in obesogenic processes related with the menopausal transition in women. Weight gain as a cardiovascular risk factor is a frequent feature in menopause women. The adipogenic effect of ascorbic acid shown in previous reports67 could aggravate obesity in the menopausal women; however, the lipolytic effect of ascorbic acid as an antioxidant might cancel this adipogenic effect. The findings in this paper have shown that the adipogenic effect overwhelmed the lipolytic effect when ascorbic acid was administered during the early period after OVX, while the lipolytic effect only lasted and reduced fat mass and body fat ratio when administered during the late period. When taking into account these results, along with those from previous reports, we suggest that ascorbic acid should not be recommended during the early stage of menopause, but could be recommended for obese post-menopausal women. Further clinical studies need to be performed to clarify the relationship between ascorbic acid and female-specific obesity.
Figures and Tables
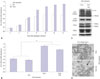 | Fig. 1Long-term effect of ASC on adipogenesis of 3T3-L1 mouse preadipocytes. (A) Graphical demonstration of long-term adipogenesis with or without ASC by ORO staining. Adipogenic induction on Day 0 and ASC treatment during the early period (Day -2, Day 0, and Day 2). (B) Effect of ASC on long-term adipogenesis depending on the treatment period. ORO staining was measured on Day 14. ASC treatment during the early period, the late period (Day 8, Day 10, and Day 12), or both (n=9). (C) Western blotting analysis of ASC treated adipocytes on Day 14 against collagens, α1(I) and α1(VI), and adipogenic markers, PPARγ and CEBPα. ASC treatment during the early period or both early and late periods. (D) Immunocytochemical features of ASC treated adipocytes against type I collagen at Day 14. ASC treatment during the early period, or both early and late periods. Arrow indicates the area of unstained cells. *p<0.05, **p<0.01. ASC, ascorbic acid; ORO, Oil Red O; OD, optical density. |
 | Fig. 2Inhibitory effect of the antioxidant ramalin on adipogenesis of 3T3-L1 mouse preadipocytes. Adipogenesis was induced with or without ASC during the early period (Day -2, Day 0, and Day 2). Ramalin treatment during the early or late period (Day 8, Day 10, and Day 12). (A) ORO staining picture of ramalin treated adipocytes on Day 14. (B) Graphical demonstration of adipogenesis in ramalin-treated adipocytes with ORO staining on Day 14 (n=9). *p<0.05, **p<0.01. ASC, ascorbic acid; ORO, Oil Red O; OD, optical density. |
 | Fig. 3Body composition analysis of ovariectomized rats with or without ASC injection. ASC injection during the early period (Week 1 and 2), the late period (Week 5 and 6), or both after OVX (n=8). (A) Body weight difference 6 weeks after OVX. (B) Body fat difference 6 weeks after OVX. *p<0.05, **p<0.01. ASC, ascorbic acid; OVX, ovariectomy. |
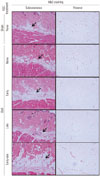 | Fig. 4Histological evaluation of ovariectomized rats with or without ASC injection. ASC injection during the early period (Week 1 and 2), late period (Week 5 and 6), or both after OVX. H&E staining was performed with subcutaneous fat and visceral fat. Arrows indicate subcutaneous fat layer. ASC, ascorbic acid; OVX, ovariectomy; H&E, hematoxylin and eosin. |
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF-2014R1A1A2058114) and was also supported by a Korea University Grant.
References
1. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Clin Obstet Gynecol. 2007; 50:354–361.

2. Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr, Roger VL, Melton LJ 3rd, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009; 16:15–23.

3. McCarthy AM, Menke A, Ouyang P, Visvanathan K. Bilateral oophorectomy, body mass index, and mortality in U.S. women aged 40 years and older. Cancer Prev Res (Phila). 2012; 5:847–854.

4. Singh PN, Haddad E, Knutsen SF, Fraser GE. The effect of menopause on the relation between weight gain and mortality among women. Menopause. 2001; 8:314–320.

5. Barnes MJ. Function of ascorbic acid in collagen metabolism. Ann N Y Acad Sci. 1975; 258:264–277.

6. Kim B, Choi KM, Yim HS, Lee MG. Ascorbic acid enhances adipogenesis of 3T3-L1 murine preadipocyte through differential expression of collagens. Lipids Health Dis. 2013; 12:182.

7. Ono M, Aratani Y, Kitagawa I, Kitagawa Y. Ascorbic acid phosphate stimulates type IV collagen synthesis and accelerates adipose conversion of 3T3-L1 cells. Exp Cell Res. 1990; 187:309–314.

8. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003; 22:18–35.

9. Zhu LL, Cao J, Sun M, Yuen T, Zhou R, Li J, et al. Vitamin C prevents hypogonadal bone loss. PLoS One. 2012; 7:e47058.

10. Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Yano M. High Vitamin C intake with high serum β-cryptoxanthin associated with lower risk for osteoporosis in post-menopausal Japanese female subjects: Mikkabi Cohort Study. J Nutr Sci Vitaminol (Tokyo). 2016; 62:185–191.

11. Kim DE, Cho SH, Park HM, Chang YK. Relationship between bone mineral density and dietary intake of β-carotene, vitamin C, zinc and vegetables in postmenopausal Korean women: a cross-sectional study. J Int Med Res. 2016; 44:1103–1114.

12. Garcia-Diaz DF, Lopez-Legarrea P, Quintero P, Martinez JA. Vitamin C in the treatment and/or prevention of obesity. J Nutr Sci Vitaminol (Tokyo). 2014; 60:367–379.

13. Hasegawa N, Niimi N, Odani F. Vitamin C is one of the lipolytic substances in green tea. Phytother Res. 2002; 16:Suppl 1. S91–S92.

14. Ji H, Om AD, Yoshimatsu T, Umino T, Nakagawa H, Sakamoto S. Effect of dietary ascorbate on lipogenesis and lipolysis activities in black sea bream, Acanthopagrus schlegelii. Fish Physiol Biochem. 2010; 36:749–755.

15. Campión J, Milagro FI, Fernández D, Martínez JA. Diferential gene expression and adiposity reduction induced by ascorbic acid supplementation in a cafeteria model of obesity. J Physiol Biochem. 2006; 62:71–80.

16. Ponec M, Weerheim A, Kempenaar J, Mulder A, Gooris GS, Bouwstra J, et al. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J Invest Dermatol. 1997; 109:348–355.

17. Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012; Chapter 5:Unit5.61.

18. Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E315–E322.

19. Wegorzewska IN, Walters K, Weiser MJ, Cruthirds DF, Ewell E, Larco DO, et al. Postovariectomy weight gain in female rats is reversed by estrogen receptor alpha agonist, propylpyrazoletriol. Am J Obstet Gynecol. 2008; 199:67.e1–67.e5.
20. Paudel B, Bhattarai HD, Koh HY, Lee SG, Han SJ, Lee HK, et al. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine. 2011; 18:1285–1290.

21. Kim SY, Jang YJ, Park B, Yim JH, Lee HK, Rhee DK, et al. Ramalin inhibits differentiation of 3T3-L1 preadipocytes and suppresses adiposity and body weight in a high-fat diet-fed C57BL/6J mice. Chem Biol Interact. 2016; 257:71–80.

22. Naylor GJ, Grant L, Smith C. A double blind placebo controlled trial of ascorbic acid in obesity. Nutr Health. 1985; 4:25–28.

23. Johnston CS, Beezhold BL, Mostow B, Swan PD. Plasma vitamin C is inversely related to body mass index and waist circumference but not to plasma adiponectin in nonsmoking adults. J Nutr. 2007; 137:1757–1762.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download