1. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006; 48:193–202. PMID:
16814667.
2. Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008; 118:1138–1145. PMID:
18725485.
3. Nasuno T, Tokura M, Kageyama M, Toyoda S, Sakuma M, Komatsu T, et al. The wound healing response after implantation of a drug-eluting stent is impaired persistently in the long term. Heart Vessels. 2016; 31:985–989. PMID:
25939630.

4. Byrne RA, Kastrati A, Kufner S, Massberg S, Birkmeier KA, Laugwitz KL, et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J. 2009; 30:2441–2449. PMID:
19720642.

5. Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008; 372:1163–1173. PMID:
18765162.

6. Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012; 33:1214–1222. PMID:
22447805.

7. Grube E, Buellesfeld L. BioMatrix Biolimus A9-eluting coronary stent: a next-generation drug-eluting stent for coronary artery disease. Expert Rev Med Devices. 2006; 3:731–741. PMID:
17280537.
8. Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009; 57:567–584. PMID:
19838148.
9. Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007; 27:1500–1510. PMID:
17510464.
10. Smits PC, Hofma S, Togni M, Vázquez N, Valdés M, Voudris V, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013; 381:651–660. PMID:
23374650.

11. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Smits PC, et al. Clinical outcomes with bioabsorbable polymer-versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014; 63:299–307. PMID:
24211507.
12. Tantawy A, Ahn CM, Shin DH, Kim JS, Kim BK, Ko YG, et al. Nobori-Biolimus-eluting stents versus Resolute Zotarolimus-eluting stents in patients undergoing coronary intervention: a propensity score matching. Yonsei Med J. 2017; 58:290–295. PMID:
28120558.

13. Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014; 11:597–606. PMID:
25154978.
14. Lüscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003; 108:1655–1661. PMID:
14517152.
15. Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004; 44:2293–2300. PMID:
15607389.

16. Mahmud E, Bromberg-Marin G, Palakodeti V, Ang L, Creanga D, Demaria AN. Clinical efficacy of drug-eluting stents in diabetic patients: a meta-analysis. J Am Coll Cardiol. 2008; 51:2385–2395. PMID:
18565394.
17. Kang SH, Park KH, Ahn HS, Park KW, Hong YJ, Koo BK, et al. Everolimus-eluting versus sirolimus-eluting coronary stents in patients with and without diabetes mellitus. EuroIntervention. 2014; 10:74–82. PMID:
24345385.

18. Kubo T, Akasaka T, Tanimoto T, Takano M, Seino Y, Nasu K, et al. Assessment of vascular response after drug-eluting stents implantation in patients with diabetes mellitus: an optical coherence tomography sub-study of the J-DESsERT. Heart Vessels. 2016; 31:465–473. PMID:
25630713.

19. Kim BK, Hong MK, Shin DH, Kim JS, Ko YG, Choi D, et al. Optical coherence tomography analysis of strut coverage in biolimus- and sirolimus-eluting stents: 3-month and 12-month serial follow-up. Int J Cardiol. 2013; 168:4617–4623. PMID:
23932862.

20. Park KH, Jeong MH, Kim JM, Park DS, Kim JH, Lim KS, et al. The impact of triple anti-platelet therapy for endothelialization and inflammatory response at overlapping bioabsorbable polymer coated drug-eluting stents in a porcine coronary model. Int J Cardiol. 2013; 168:1853–1858. PMID:
23347613.

21. Nakazawa G, Shinke T, Ijichi T, Matsumoto D, Otake H, Torii S, et al. Comparison of vascular response between durable and biodegradable polymer-based drug-eluting stents in a porcine coronary artery model. EuroIntervention. 2014; 10:717–723. PMID:
25330504.

22. Lim KS, Jeong MH, Bae IH, Park DS, Kim JM, Kim JH, et al. Histopathological comparison among biolimus, zotarolimus and everolimus-eluting stents in porcine coronary restenosis model. Korean Circ J. 2013; 43:744–751. PMID:
24363750.

23. de Waha A, Stefanini GG, King LA, Byrne RA, Serruys PW, Kufner S, et al. Long-term outcomes of biodegradable polymer versus durable polymer drug-eluting stents in patients with diabetes a pooled analysis of individual patient data from 3 randomized trials. Int J Cardiol. 2013; 168:5162–5166. PMID:
23993323.

24. Räber L, Kelbæk H, Taniwaki M, Ostojic M, Heg D, Baumbach A, et al. Biolimus-eluting stents with biodegradable polymer versus bare-metal stents in acute myocardial infarction: two-year clinical results of the COMFORTABLE AMI trial. Circ Cardiovasc Interv. 2014; 7:355–364. PMID:
24847017.
25. Tomai F, De Luca L, Altamura L, Versaci F, Pennacchi M, Proietti I, et al. One-year outcome from an all-comers population of patients with ST-segment elevation myocardial infarction treated with biolimus-eluting stent with biodegradable polymer. Catheter Cardiovasc Interv. 2015; 85:352–358. PMID:
25115927.

26. Zhang YJ, Iqbal J, Windecker S, Linke A, Antoni D, Sohn HY, et al. Biolimus-eluting stent with biodegradable polymer improves clinical outcomes in patients with acute myocardial infarction. Heart. 2015; 101:271–278. PMID:
25423953.

27. Cockburn J, Pareek N, Poliacikova P, Saraf S, Williams R, Dhillon G, et al. Clinical outcomes with 6 months dual antiplatelet therapy after implantation of biolimus-A9 drug eluting coronary stents. Int J Cardiol. 2014; 172:185–189. PMID:
24462139.

28. Camenzind E, Boersma E, Wijns W, Mauri L, Rademaker-Havinga T, Ordoubadi FF, et al. Modifying effect of dual antiplatelet therapy on incidence of stent thrombosis according to implanted drugeluting stent type. Eur Heart J. 2014; 35:1932–1948. PMID:
24627416.
29. Colombo A, Chieffo A. Dual antiplatelet therapy after drug-eluting stents--how long to treat. N Engl J Med. 2014; 371:2225–2226. PMID:
25399657.
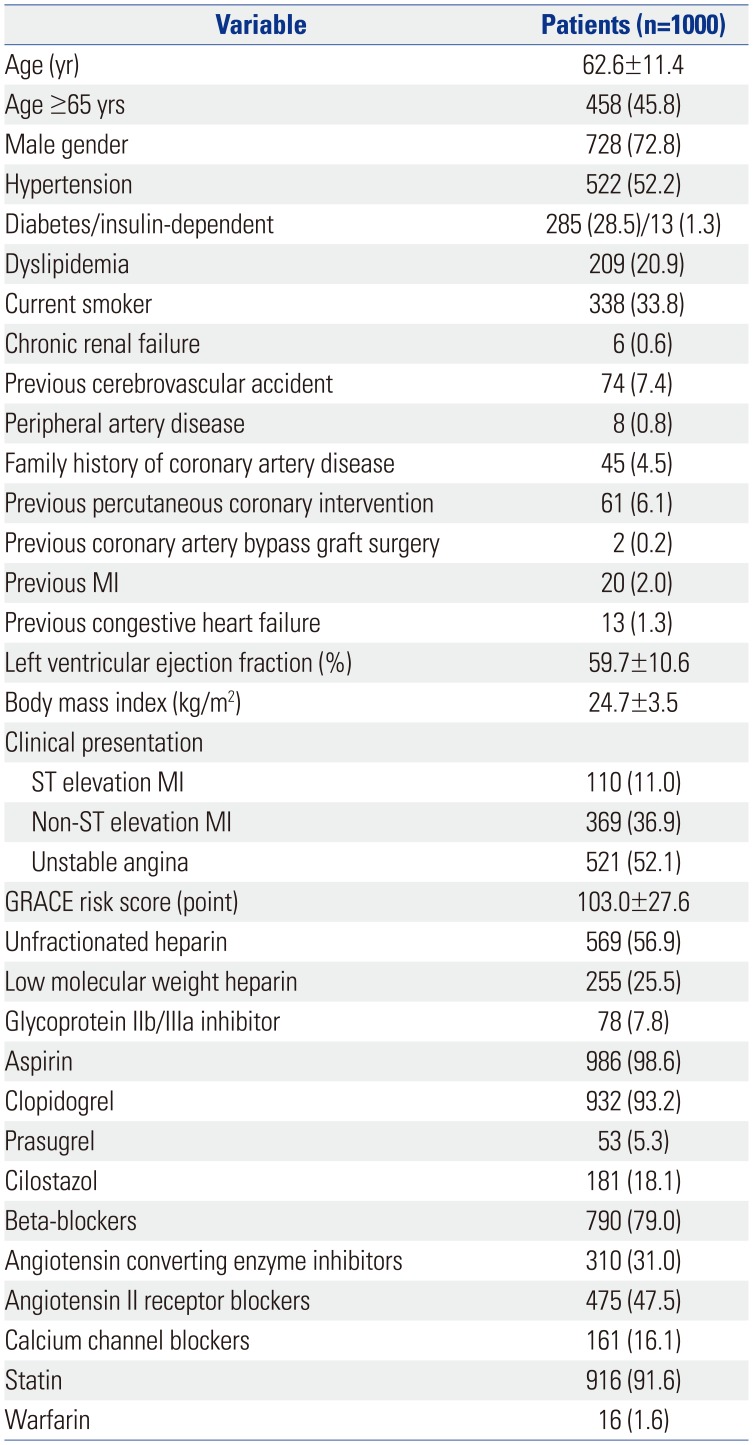
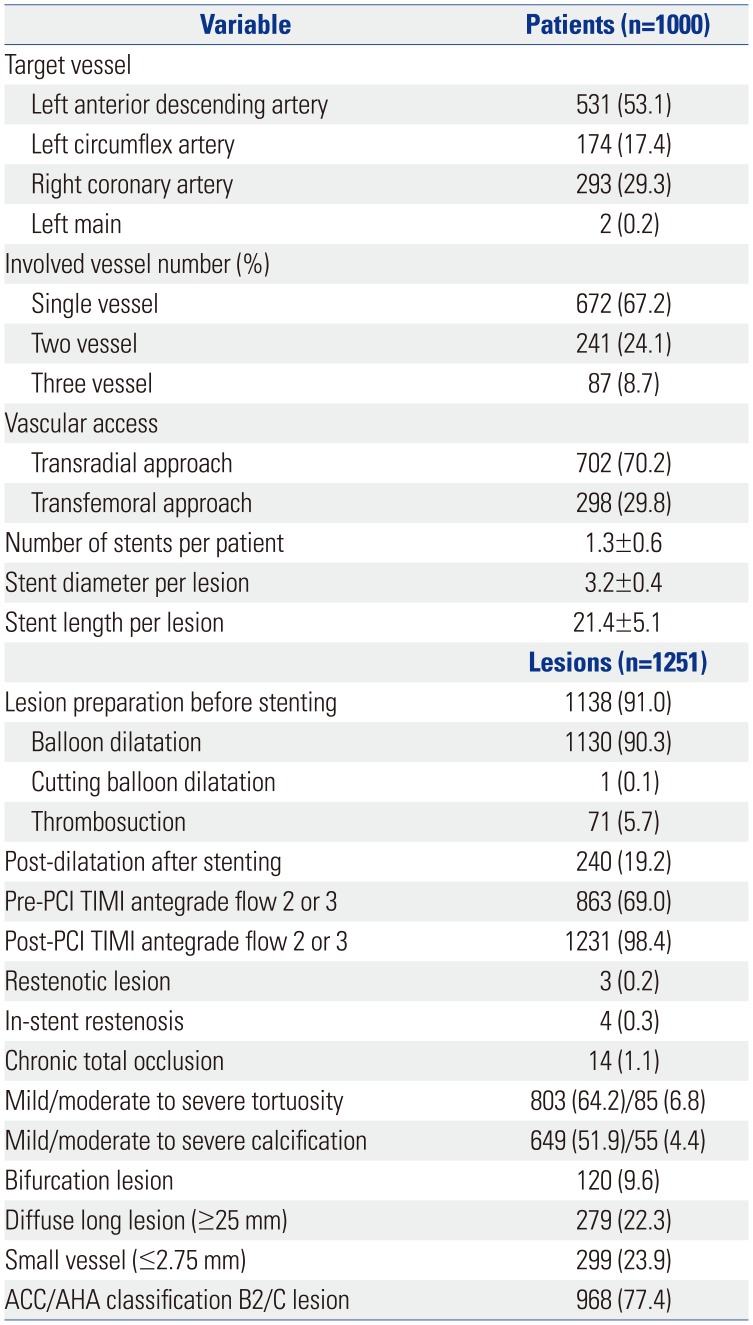
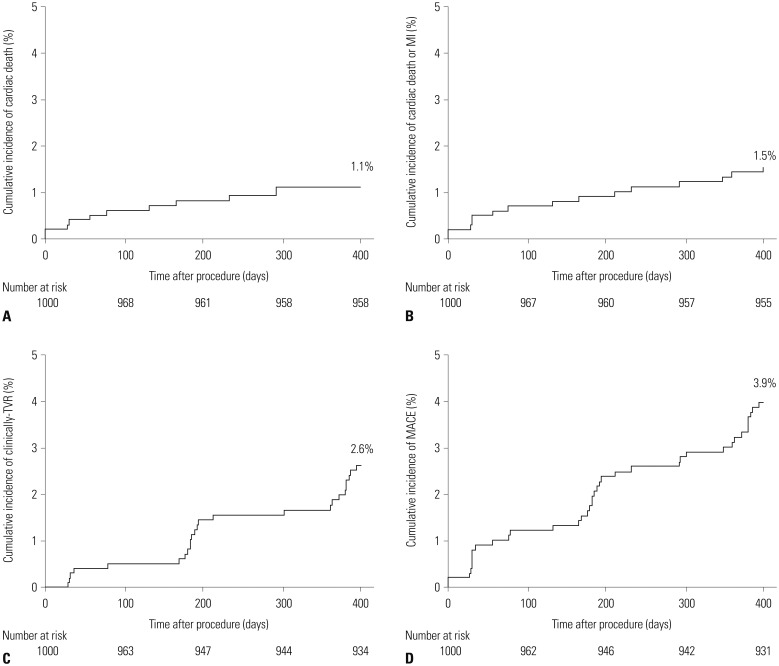
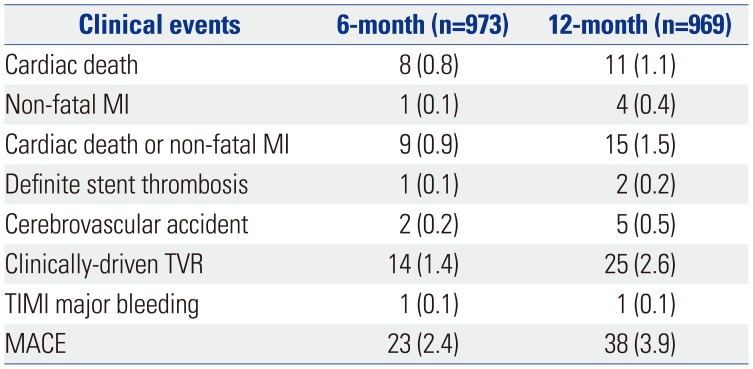





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download