Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1Classification of cases (n=790) into the muscularity group (in red) and non-muscularity group (in blue) according to the plot of SMM to BW using the linear support vector machines classification method. SMM, skeletal muscle mass; BW, body weight. |
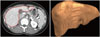
 | Fig. 3Extraction of abdomen, subcutaneous fat, and visceral fat from the umbilicus CT image. (A) Extracted abdomen (red). (B) Extracted subcutaneous fat (green) and visceral fat (red). |
Table 1
Summary of SLV Estimation Formulas

| Studies | SLV regression formulas (gender: female=0, male=1) | adj. R2 | Data source | Population | Sample size | Age (range; years) | Mean percentage error (%)* | RMSE |
|---|---|---|---|---|---|---|---|---|
| Urata, et al.13 | SLV=2.4+706.2×BSA | 0.96 | CT volumetry | Japanese | 96 | 11.1±8.8 | −12.3 | 291.3 |
| Heinemann, et al.14 | SLV=−345.7+1072.8×BSA | 0.30 | Autopsy | German | 1332 | 50.6±18.9 | 10.1 | 287.3 |
| Vauthey, et al.15 | SLV=−794.41+1267.28×BSA | 0.46 | CT volumetry | North American & European | 292 | 54 (14–90) | 6.3 | 239.0 |
| Hashimoto, et al.16 | SLV=−404.8+961.3×BSA | 0.58 | CT volumetry | Japanese | 301 | 17–66 | −7.6 | 261.0 |
| Yu, et al.17 | SLV=21.585×BW0.732×BH0.225 | 0.59 | Autopsy | Korean | 652 | 42.4±16.5 | 8.2 | 253.2 |
| Yuan, et al.18 | SLV=−274.7+949.7×BSA-48.3×age factor (1: <40, 2: 41–60, 3: >60) | 0.44 | CT volumetry | Chinese | 112 | 48.7±12.4 | −3.2 | 256.1 |
| Fu-Gui, et al.19 | SLV=334.024+11.508×BW | 0.36 | Graft measurement | Chinese | 115 | 36.0±9.6 (19–57) | −19.6 | 367.3 |
| Poovathumkadavil, et al.20 | SLV=555.65+12.255×BW | 0.37 | CT volumetry | Saudia Arabian | 351 | 49.2±16.1 | −1.2 | 251.2 |
| Kokudo, et al.21 | SLV=203.3-3.61×age+58.7×TW-463.7×race (1=Japanese, 0=Swiss) | N/A | CT volumetry | Japanese & Swiss | Japanese: 180 Swiss: 160 | Japanese: 39.4 (20–65), Swiss: 56.5 (19–90) | N/A | 171.8 |
| Um, et al.22 | SLV=−439.169+893.485×BSA | 0.52 | CT volumetry | Korean | 1000 | 28.1±8.8 | N/A | N/A |
Table 2
Summary of Sample Sizes of Data Sets Formed from the Original Data Set (n=790) Used for Statistical Model Development and Cross-Validation

Table 3
Regression Formulas for SLV Estimation

SLV, standard liver volume; AE, absolute error; PAE, percentage of absolute error; BW, body weight; BSA, body surface area; SMM, skeletal muscle mass; WC, waist circumference; BFP, body fat percentage; AFP, abdominal fat percentage; SFA, subcutaneous fat area.
Combined data: n=494 for model development and n=80 for model cross-validation; muscularity group data: n=134 for model development and n=30 for model cross-validation; non-muscularity group data: n=360 for model development and n=50 for model cross-validation.
*Preferred formulas depending on data availability.
Table 4
Comparison of the SLV Estimation Formulas Proposed in the Present Study with Existing Formulas Using a Validation Data set of n=80 (30 from Muscularity Group and 50 from Non-Muscularity Group)

| Study | SLV regression formulas (gender: female=0, male=1) | AE (mL) | PAE (%) | Percentage of PAE >20% (%) | PE (%) |
|---|---|---|---|---|---|
| Present study | |||||
| Combined group | SLV=331−4.1×age+41.6×gender+15.3×BW | 166.8±127.1 | 12.7±9.0 | 17.5 | −0.6±15.5 |
| Muscularity group | |||||
| Abdominal geometry data unavailable | SLV=161−3.6×age−182×gender+27.4×SMM | 158.2±98.6 | 12.4±7.6 | 20.0 | 0.5±14.6 |
| Abdominal geometry data available | SLV=45−4.3×age−152×gender+24.3×SMM+3.36×WC | 156.1±104.4 | 12.3±8.2 | 16.7 | 0.9±14.7 |
| Non-muscularity group | |||||
| Abdominal geometry data unavailable | SLV=−412−4.3×age+13.6×BW−6.0×BFP+1174×AFP | 135.6±97.8 | 11.2±9.0 | 12.0 | 0.4±14.4 |
| Abdominal geometry data available | SLV=−1063−5.6×age−93.0×gender+11.7×BW−4.9×BFP+1211×AFP+12.9×WC−1.8×SFA | 116.2±98.5 | 9.6±8.9 | 14.0 | 0.0±13.1 |
| Urata, et al.13 | SLV=2.4+706.2×BSA | 171.0±125.7 | 13.3±9.5 | 22.5 | 1.9±16.2 |
| Heinemann, et al.14 | SLV=−345.7+1072.8×BSA | 333.7±178.8 | 28.3±18.3 | 63.8 | 27.1±20.0 |
| Vauthey, et al.15 | SLV=−794.41+1267.28×BSA | 267.4±158.0 | 22.5±15.5 | 53.8 | 19.6±19.1 |
| Hashimoto, et al.16 | SLV=−404.8+961.3×BSA | 181.1±119.1 | 14.6±10.4 | 26.3 | 6.4±16.8 |
| Yu, et al.17 | SLV=21.585×BW0.732×BH0.225 | 290.7±165.5 | 24.6±16.5 | 57.5 | 22.7±19.0 |
| Yuan, et al.18 | SLV=−274.7+949.7×BSA−48.3×age factor (1: <40, 2: 41–60, 3: >60) | 195.8±121.1 | 16.2±11.6 | 27.5 | 10.0±17.2 |
| Fu-Gui, et al.19 | SLV=334.024+11.508×BW | 192.1±170.0 | 13.5±9.8 | 28.8 | −9.0±14.0 |
| Poovathumkadavil, et al.20 | SLV=555.65+12.255×BW | 211.1±126.0 | 17.7±12.7 | 38.8 | 12.8±17.6 |
| Kokudo, et al.21 | SLV=203.3−3.61×age+58.7×TW−463.7×race (1=Asian, 0=Caucasian) | 170.8±136.1 | 13.0±9.8 | 20.0 | −1.6±15.5 |
| Um, et al.22 | SLV=−439.169+893.485×BSA | 179.4±154.9 | 12.9±9.5 | 25.0 | −6.1±14.8 |
SLV, standard liver volume; AE, absolute error; PAE, percentage of absolute error; PE, percentage of error; BW: body weight, SMM: skeletal muscle mass, WC: waist circumference, BFP: body fat percentage, AFP: abdominal fat percentage, SFA: subcutaneous fat area, BSA: body surface area, BH: body height, TW: thoracic width.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download