Abstract
Purpose
14-3-3ζ regulates cell signaling, cell cycle progression, and apoptosis, and its overexpression is associated with disease recurrence and poor clinical outcomes in some solid tumors. However, its clinicopathological role in ovarian cancer is unknown. Our goal was to investigate whether 14-3-3ζ is associated with ovarian cancer prognosis.
Materials and Methods
We examined 14-3-3ζ expression by immunohistochemistry in ovarian cancer tissues obtained from 88 ovarian cancer patients. The examined tissues were of various histologies and stages. 14-3-3ζ expression was also analyzed by western blot in seven ovarian cancer cell lines and a primary ovary epithelial cell line. Cell viability was measured using an MTS-based assay following cisplatin treatment.
Results
Among the ovarian cancer samples, 53.4% (47/88) showed high 14-3-3ζ expression, and 14-3-3ζ overexpression was positively correlated with more advanced pathologic stages and grades. 14-3-3ζ overexpression was also significantly associated with poor disease-free survival (DFS) and overall survival (OS) of ovarian cancer patients. Median DFS and OS were 1088 and 3905 days, respectively, in the high 14-3-3ζ expression group, but not reached in the low 14-3-3ζ expression group (p=0.004 and p=0.033, log-rank test, respectively). Downregulating 14-3-3ζ by RNA interference in ovarian cancer cells led to enhanced sensitivity to cisplatin-induced cell death.
Among gynecological cancers, ovarian cancer causes the majority of deaths in women. Because of nonspecific early symptoms and unreliable screening measures, most patients present with late-stage disease and thus a poor prognosis for long-term survival.1 Currently, standard treatment involves maximal debulking, followed by combination chemotherapy consisting of a platinum-based compound and a taxane.2 Although patients may initially have positive responses, most eventually develop multidrug resistance and die from progressive disease.3
The different 14-3-3 isoforms are ubiquitously expressed and highly conserved in all eukaryotic organisms.4 Seven mammalian isoforms (β, γ, ε, η, σ, τ, and ζ) have been identified. 14-3-3 proteins form heterodimers or homodimers and bind to specific phosphorylation motifs on target proteins, altering their subcellular localization, stability, and enzymatic activity.567 By modulating the activity of their binding partners, 14-3-3 proteins have been shown to regulate diverse cellular processes, including apoptosis, mitogenic and stress signaling, cell cycle progression, transcription, metabolism, and cytoskeletal integrity.89 Among the 14-3-3 isoforms, 14-3-3ζ has been reported to be associated with tumorigenesis and poor clinical outcomes, and knockdown studies using small interfering RNA have shown that its reduced expression increases the efficacy of chemotherapeutic agents in some solid tumors.10 It has been reported that increased 14-3-3ζ expression is positively correlated with more advanced pathologic stages and grades of non-small cell lung cancer, breast cancer, and adenocarcinoma of the esophagogastric junction.111213 However, the clinical relevance of 14-3-3ζ expression in ovarian cancer remains unknown. Therefore, the purpose of this study was to investigate whether 14-3-3ζ is associated with ovarian cancer prognosis.
To determine the correlation between 14-3-3ζ expression and clinicopathological features in ovarian cancer, archived paraffin-embedded and frozen tumor tissues were retrospectively obtained from Ewha Womans University Mokdong Hospital and Ewha Biospecimen Bank, and ovarian cancer patients in this study were diagnosed and treated at Ewha Womans University Mokdong Hospital, Republic of Korea. We included only epithelial ovarian cancer patients who underwent primary debulking surgery followed by chemotherapy. In our hospital, optimal debulking is defined as no visible or residual tumors up to 5 mm. All the cases included in this study reached optimal debulking state. The relevant clinical and survival data were available for all patients. Written informed consent was obtained from all patients, and this study was approved by our hospital's Institutional Review Board (EUMC 2017-01-032-002). Immunohistochemistry (IHC) was performed using a rabbit polyclonal antibody against 14-3-3ζ (Santa Cruz Biotechnology, Dallas, TX, USA). Briefly, 4-µm sections were transferred to adhesive slides and dried at 62℃ for 30 min. After incubation with primary antibody, immunodetection was achieved by adding biotinylated anti-rabbit immunoglobulin followed by peroxidase-labeled streptavidin; 3,3′-diaminobenzidine was used as the chromogenic substrate. Negative controls were not incubated with a primary antibody. Slides were counterstained with Harris hematoxylin. All IHC data were interpreted by two pathologists (S.H. Sung and M.S. Cho), and staining intensity was scored using a four-tier system: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining.
The cell lines used in this study were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in RPMI-1640 (SH30027; Hyclone, Marlborough, MA, USA) supplemented with 10% fetal bovine serum (12483-020; Gibco, Waltham, MA, USA) in a 5% CO2 atmosphere at 37℃. The immortalized human ovary epithelial cell line (HOSE2) was obtained as a gift from Dr. Tohru Kiyono (National Cancer Center, Tokyo, Japan).14 To establish stable 14-3-3ζ-knockdown cell lines and corresponding controls, lentiviral particles containing 14-3-3ζ or control short hairpin RNA were transduced into TOV21G ovarian cancer cells, and stable cells were selected with puromycin (Sigma Aldrich, St. Louis, MO, USA). Lentiviral particles were purchased from Santa Cruz Biotechnology.
Protein lysates were prepared using a PRO-PREP protein extraction solution (Cat#17081; iNtRON Biotechnology, Seongnam, Korea) according to the manufacturer's protocol. Equal amounts of protein were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After blocking with 5% nonfat skim milk, membranes were blotted with the designated primary and secondary antibodies, developed using Clarity™ Western ECL Substrate (#170-5060, BIO-RAD, Hercules, CA, USA), and visualized with an ImageQuant LAS4000 Mini biomolecular imager (GE Healthcare, Marlborough, MA, USA). β-actin was used as an internal loading control. Antibodies against 14-3-3ζ and β-actin were obtained from Santa Cruz Biotechnology.
Cells were seeded at 3000 cells/well in 96-well plates and treated with cisplatin for 72 h. Viable cells were measured using an MTS-based assay, CellTiter 96 Aqueous One Solution (G3580; Promega, Madison, WI, USA) following the manufacturer's protocol. The absorbance of each well was measured on a Versa-Max ELISA Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) at 490 nm. The proportion of viable cells in the treatment group was normalized to that of control wells.
Overall survival (OS) times were calculated from the date of first surgery to the date of death from any cause. Disease-free survival (DFS) times were calculated from the date of primary surgery to the date when the first local or regional recurrence was diagnosed or the date of death associated with disease. Statistical analyses were performed using IBM SPSS Statistics software ver. 23.0 (IBM Corp., Armonk, NY, USA). Chi-square analysis was used for comparisons between 14-3-3ζ intensity and categorical variables in Table 1. Survival analyses were performed using the Kaplan-Meier method, and statistical differences were assessed using the log-rank test. Univariate and multivariate analyses were performed using the ratio test of the Cox's proportional hazards model. In multivariate survival analysis, surgical stage and histologic grade were not adjusted to avoid invalid statistical results by overmatching. p-values less than 0.05 were considered significant and determined by log-rank analysis. Mean differences between two groups were analyzed using two-tailed Student's t-tests.
To investigate the association of 14-3-3ζ with ovarian cancer, we first examined whether 14-3-3ζ is overexpressed in ovarian cancer cell lines. The results showed that 14-3-3ζ expression was highly increased in seven ovarian cancer cell lines (A2780, A2780-CisR, TOV21G, TOV21G-CisR, ES-2, SCOV3, and OV-CAR3), compared with an immortalized human ovary epithelial cell line (HOSE2) (Fig. 1A). Consistent with these results, 14-3-3ζ levels were upregulated in the majority of ovarian cancer tissues, compared with benign tumor tissues (Fig. 1B).
To investigate the clinicopathological relevance of 14-3-3ζ expression in ovarian cancer, we next performed IHC on cancer tissues obtained from 88 ovarian cancer patients with different clinical stages, histologic types, and grades. We scored 14-3-3ζ IHC staining as negative (score 0), weakly positive (score 1), moderately positive (score 2), and strongly positive (score 3) based on the percentage of positively stained cells and staining intensity. Samples with strongly positive staining (score 3) were defined as 14-3-3ζ overexpression or high expression. No specimens scored negative among the 88 cases; all tumor samples showed positive 14-3-3ζ staining (Table 1, Fig. 2A). The relationships between 14-3-3ζ expression and clinicopathological characteristics are summarized in Table 1. 14-3-3ζ staining was primarily observed in the cytoplasm of ovarian cancer cells, although nuclear staining was also found in many strong positive cells; adjacent stromal cells were negative or weakly positive (Fig. 2A). 14-3-3ζ overexpression was found in 47 of 88 (53.4%) ovarian cancers and occurred in 39 of 60 (65%) serous samples, compared with only eight of 28 (28.6%) non-serous samples (Table 1). Although the cohort of non-serous histologic types was small, a significant difference in 14-3-3ζ overexpression was found between the serous and non-serous histologic types (p=0.003) (Table 1). In multivariate analysis, 14-3-3ζ overexpression was significantly associated with more advanced stages and grades in ovarian cancer (p=0.022 and p<0.001, respectively) (Table 1). Next, we examined the relationship between 14-3-3ζ overexpression and DFS. Notably, 14-3-3ζ overexpression showed a significant correlation with reduced DFS in the 88 ovarian cancer patients (Fig. 2B). While median DFS was not reached in the low 14-3-3ζ expression group, median DFS of the high 14-3-3ζ expression group was 1088 days (95% CI 104.8–2071.2) (p=0.004 by log-rank test). In accordance, high 14-3-3ζ expression was also significantly associated with reduced OS (Fig. 2C). Median time for OS was not reached in the low expression group and was 3905 days (95% CI 1789.4–6020.5) in the high expression group (p=0.033 by log-rank test). Taken together, these results show that 14-3-3ζ overexpression was significantly correlated with more advanced pathological stages, grades, and poor clinical outcomes in ovarian cancer patients, suggesting that 14-3-3ζ might play an important role in ovarian cancer progression.
Cisplatin is a standard therapy for ovarian cancer; therefore, we investigated whether 14-3-3ζ knockdown could enhance the sensitivity of ovarian cancer cells to cisplatin. To this end, TOV21G ovarian cancer cells were infected with high titers of lentiviral particles encoding shRNAs against 14-3-3ζ, and puromycin-selected colonies were pooled to diminish variations between selected colonies. As shown in Fig. 3A, 14-3-3ζ was effectively silenced in the stable cell line, compared with non-infected and control shRNA transduced TOV21G cells. We assessed the effects of reduced 14-3-3ζ expression on cell growth by comparing the 14-3-3ζ knockdown cells with control cells in proliferation assays. We did not observe a significant difference in the cell growth rate between TOV21G-sh14-3-3ζ cells and control cells (data not shown). Next, we evaluated whether 14-3-3ζ knockdown could alter the sensitivity of TOV21G ovarian cancer cells to cisplatin. TOV21G-sh14-3-3ζ and the corresponding TOV21G-shCont cells were exposed to cisplatin for 72 h, and cell viability was examined. As the concentration of cisplatin increased, the survival rate of both cell lines decreased (Fig. 3B). Although there was no significant difference in cell viability between both cell lines at the concentration of 2.5 µM cisplatin, the inhibition of cell viability was significantly enhanced by the treatment of 5 µM cisplatin in the TOV21G-sh14-3-3ζ cells, compared with control cells (Fig. 3B), indicating that 14-3-3ζ knockdown might increases the sensitivity of ovarian cancer cells to cisplatin.
The incidence rate of ovarian cancer in Korea is known to be much lower than that in Western countries. However, recent studies have reported that the incidence of ovarian cancer has been increasing gradually and was estimated to comprise 17% (2374) of all newly diagnosed woman's cancers during 2015 in Korea.15 Among gynecologic cancers, ovarian cancer shows the poorest survival rate, owing to the difficulty of early diagnosis and high recurrence rate after treatment, requiring proper biomarkers for diagnosis and prognosis for ovarian cancer.
Recent studies have revealed oncogenic functions and an association between 14-3-3ζ and poor DFS and OS in breast, lung, prostate, and head and neck cancers.111216 However, to date, there have been no reports regarding the clinical significance of 14-3-3ζ expression in ovarian cancer. This prompted us to determine the clinicopathological and prognostic significance of 14-3-3ζ overexpression in ovarian cancer. In this study, we first showed that high 14-3-3ζ expression was significantly associated with poor prognosis in ovarian cancer patients. Furthermore, knocking down 14-3-3ζ by shRNA significantly increased the sensitivity of ovarian cancer cells to cisplatin, suggesting that 14-3-3ζ might play an important role in ovarian cancer progression and resistance to cisplatin.
A pivotal role for 14-3-3ζ in tumor progression has been suggested in previous studies, which showed that 14-3-3ζ knockdown reduced anchorage-independent tumor cell growth in breast and lung cancer models.1217 Furthermore, silencing 14-3-3ζ in combination with chemotherapeutic agents led to increased cancer cell death.1011 Although we did not observe a reduction in the cell growth rate or anchorage-independent growth after knocking down 14-3-3ζ in TOV21G cells (data not shown), our data showed that 14-3-3ζ knockdown could increase the sensitivity of ovarian cancer cells to cisplatin (Fig. 3B). These results imply that 14-3-3ζ might be involved in chemoresistance rather than acting as an oncogenic driver in ovarian cancer. The mechanism by which 14-3-3ζ knockdown enhances cisplatin sensitivity is currently unknown. Further studies are required to reveal resistance mechanisms of 14-3-3ζ to anticancer drugs such as cisplatin to enhance drug sensitivity in ovarian cancer. However, our results suggest that targeting 14-3-3ζ could improve the efficacy of cisplatin in ovarian cancer.
Recurrence is a common event in ovarian cancer; approximately 70% of ovarian cancer patients experience recurrence. Because recurrent ovarian cancer is typically an incurable disease, adjuvant treatment is the most important determinant of clinical outcomes. Early predictors of recurrence could help identify patients who would benefit from more aggressive therapies to battle recurrent cancer. Our data showed that 14-3-3ζ overexpression was significantly associated with reduced DFS (Fig. 2B). Although the precise role of 14-3-3ζ in the process of tumor progression has not yet been revealed in this study, our data indicate that 14-3-3ζ might play an important role in ovarian cancer progression including recurrence. A recent report showed that 14-3-3ζ is not only detected in tumor tissue, but is also secreted from cancer cells and can be identified in the serum of patients with head and neck small cell carcinomas.18 This suggests that 14-3-3ζ could also be applied to monitor ovarian cancer after primary treatment using non-invasive biopsies, such as serum and ascites samples from ovarian cancer patients.
It is well established that the prognostic factors for ovarian cancer include age, surgical stage, histologic grade, remnant disease, and patient performance status. All patients enrolled in this study underwent surgical staging and achieved optimal debulking. Thus, for this cohort, there were no effects from residual disease on survival. Surgical stage and histologic grade showed positive correlations with the high expression of 14-3-3ζ (Table 1). We hypothesized that 14-3-3ζ has a role in tumorigenesis and tumor progression, and inferred that high 14-3-3ζ expression might be associated with high ovarian cancer grade, which could subsequently contribute to rapid spread, resulting in more advanced-staged disease. Hence, we did not adjust surgical stage and histologic grade in the multivariate analysis because such an overmatching might have introduced invalid statistical results.
In conclusion, our findings suggest that 14-3-3ζ overexpression is associated with poor clinical outcomes, including DFS and OS, in ovarian cancer patients. 14-3-3ζ knockdown also enhances the sensitivity of ovarian cancer cells to cisplatin. Thus, 14-3-3ζ might be a potential prognostic biomarker and an attractive therapeutic target for sensitizing ovarian cancer cells to cisplatin.
Figures and Tables
 | Fig. 1High 14-3-3ζ expression in ovarian cancer cell lines and tumor tissues. (A) Western blot analysis of 14-3-3ζ expression in primary ovary epithelial (HOSE2) cells and ovarian cancer cell lines. A2780-CisR and TOV21G-CisR cells are cell lines with acquired cisplatin resistance. (B) Western blot analysis of 14-3-3ζ expression in five benign ovarian tumors and nine ovarian cancer tissues. β-actin (Actin) was used as the loading control. |
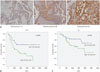 | Fig. 214-3-3ζ overexpression is associated with poor DFS and OS in ovarian cancer patients. (A) Representative 14-3-3ζ immunohistochemistry staining in ovarian cancer specimens (n=88). Tumors were classified as negative (0), weakly positive (1), moderately positive (2), and strongly positive (3) for 14-3-3ζ expression (magnification, ×100). (B and C) DFS (B) and OS (C) rates of ovarian cancer patients with low (0, 1, 2) versus high (3) 14-3-3ζ expression. p-values for B and C were determined by log-rank analysis. DFS, disease-free survival; OS, overall survival. |
 | Fig. 3Silencing 14-3-3ζ enhances the sensitivity of ovarian cancer cells to cisplatin. (A) Establishing the stable TOV21G cell line with 14-3-3ζ knockdown. Western blot analysis of 14-3-3ζ protein levels in the indicated stable cell lines and non-infected TOV21G cells. β-actin (Actin) was used as the internal loading control; n.s. indicates a non-specific band. (B) Effects of 14-3-3ζ knockdown on cell survival in ovarian cancer cells treated with cisplatin for 72 h. Viable cells were measured using an MTS-based assay. Columns represent means of six replicate experiments; bars±standard deviation; *p<0.001. |
Table 1
Clinicopathological Characteristics of Ovarian Cancer Patients

ACKNOWLEDGEMENTS
This study was supported by a grant from the Korean Health Technology R&D Project (Ministry of Health and Welfare, Korea, HI12C0050) and RP-Grant 2017 of Ewha Womans University.
We thank Dr. Tohru Kiyono who kindly gifted us the primary human ovary epithelial cell line HOSE2. We also thank James P. Mahaffey, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
1. Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009; 113:775–782.

2. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006; 354:34–43.

3. Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991; 9:389–393.

4. Aitken A, Jones D, Soneji Y, Howell S. 14-3-3 proteins: biological function and domain structure. Biochem Soc Trans. 1995; 23:605–611.

5. Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009; 19:16–23.

6. Tzivion G, Avruch J. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem. 2002; 277:3061–3064.

7. Yaffe MB. How do 14-3-3 proteins work?–Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002; 513:53–57.

8. van Hemert MJ, Steensma HY, van Heusden GP. 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays. 2001; 23:936–946.

9. Tzivion G, Gupta VS, Kaplun L, Balan V. 14-3-3 proteins as potential oncogenes. Semin Cancer Biol. 2006; 16:203–213.

10. Matta A, DeSouza LV, Ralhan R, Siu KW. Small interfering RNA targeting 14-3-3ζ increases efficacy of chemotherapeutic agents in head and neck cancer cells. Mol Cancer Ther. 2010; 9:2676–2688.

11. Fan T, Li R, Todd NW, Qiu Q, Fang HB, Wang H, et al. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007; 67:7901–7906.

12. Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009; 69:3425–3432.

13. Watanabe N, Komatsu S, Ichikawa D, Miyamae M, Ohashi T, Okajima W, et al. Overexpression of YWHAZ as an independent prognostic factor in adenocarcinoma of the esophago-gastric junction. Am J Cancer Res. 2016; 6:2729–2736.
14. Sasaki R, Narisawa-Saito M, Yugawa T, Fujita M, Tashiro H, Katabuchi H, et al. Oncogenic transformation of human ovarian surface epithelial cells with defined cellular oncogenes. Carcinogenesis. 2009; 30:423–431.

15. Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013; 24:298–302.

16. Matta A, DeSouza LV, Shukla NK, Gupta SD, Ralhan R, Siu KW. Prognostic significance of head-and-neck cancer biomarkers previously discovered and identified using iTRAQ-labeling and multidimensional liquid chromatography-tandem mass spectrometry. J Proteome Res. 2008; 7:2078–2087.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download