Abstract
Purpose
Prediabetes is an independent risk factor for cardiovascular disease. However, data on the long term adverse clinical outcomes of prediabetic patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents (DESs) are scarce.
Materials and Methods
The study population comprised 674 consecutive non-diabetic patients who underwent elective PCI between April 2007 and November 2010. Prediabetes was defined as hemoglobin A1c (HbA1c) of 5.7% to 6.4%. Two-year cumulative clinical outcomes of prediabetic patients (HbA1c of 5.7% to 6.4%, n=242) were compared with those of a normoglycemic group (<5.7%, n=432).
Results
Baseline clinical and angiographic characteristics were similar between the two groups, except for higher glucose levels (104.8±51.27 mg/dL vs. 131.0±47.22 mg/dL, p<0.001) on admission in the prediabetes group. There was no significant difference between the two groups in coronary angiographic parameters, except for a higher incidence of diffuse long lesion in the prediabetes group. For prediabetic patients, trends toward higher incidences of binary restenosis (15.6% vs. 9.8 %, p=0.066) and late loss (0.71±0.70 mm vs. 0.59±0.62 mm, p=0.076) were noted. During the 24 months of follow up, the incidence of mortality in prediabetic patients was higher than that in normoglycemic patients (5.5% vs. 1.5%, p=0.007).
Prediabetes is defined as a state of intermediate hyperglyce mia using two specific parameters, impaired fasting glucose defined as fasting plasma glucose of 110 mg/dL to 125 mg/dL and impaired glucose tolerance (IGT) defined as 2-h plasma glucose of 140-200 mg/dL after ingestion of 75 g of oral glucose load or a combination of the two based on a 2-h oral glucose tolerance test (OGTT).1 IGT and impaired fasting glycemia have been found to be markers of increased risk for the development of type 2 diabetes mellitus (DM).2 Previous studies have shown that prediabetes is an independent risk factor for cardiovascular disease3 and that prediabetes is associated with an increased risk of coronary and cardiovascular death.4
Abnormal hemoglobin A1c (HbA1c) level has been found to hold prognostic significance in non-diabetic patients undergoing percutaneous coronary intervention (PCI).56 However, data regarding the impact of prediabetes on long-term clinical outcomes in patients undergoing PCI with drug-eluting stents (DESs) are limited. In the 1st generation DES era, the incidence of major adverse cardiac events (MACE) and specific cardiovascular events during a 3-year follow-up period was reported at around 20% in prediabetic patients with sirolimus- or paclitaxel-eluting stents.7 In addition, there are no data on prediabetic patients undergoing PCI according to recent guidelines on prediabetes, HbA1c of 5.7% to 6.4%,8 with both 1st and 2nd generation DESs. The clinical impact and management of prediabetic patients undergoing PCI remain unknown. Therefore, we investigated the long-term clinical outcomes in prediabetic patients undergoing PCI with DES compared to normoglycemic patients.
From April 2007 to November 2010, all patients who underwent elective PCI with DESs had baseline laboratory studies, including HbA1c levels, completed before the procedure. PCI was performed using standard techniques, including predilation and subsequent intracoronary stenting. All patients were treated with aspirin and received 300-600 mg of clopidogrel preloaded before PCI, followed by daily administration of 75 mg that encouraged to be continued for at least 1 year. Other adjunctive pharmacotherapy was administered at the discretion of the operator. Repeat coronary angiography was performed for recurrent symptoms or objective evidence of ischemia during provocative testing. Routine angiographic follow up was not undertaken. Patients who did not undergo HbA1c tests were excluded, as well as patients who were already diagnosed with DM and treated with diet therapy, anti-diabetic drugs, or insulin therapy. Finally, 674 patients with an HbA1c level of <6.5% were included in this analysis.
Approximately 24 months after the index intervention, all patients meeting criteria for this study were contacted and invited to participate in the study. After informed consent was obtained from the patient or a family member, clinical follow up performed during an outpatient visit, telephone interviews, and hospital records were reviewed. The Medical Device Institutional Review Board (IRB) of Korea University Guro Hospital approved the study protocol (IRB Number: MD09002).
The primary end point of this study was the incidence of MACE, defined as the composite of cardiovascular mortality, myocardial infarction (MI), or need for target vessel revascularization (TVR) within 24 months of the index PCI. Secondary end points included each component of the primary end point in addition to post-procedural rehospitalization, recurrent angina, and stroke. All end point data were adjudicated by an experienced cardiovascular physician blinded to clinical details and outcomes.
DM was defined as treatment with insulin or oral hypoglycemic medications or a HbA1c level ≥6.5% in non-diabetic patients. Prediabetes was defined as HbA1c of 5.7% to 6.4%. This cutoff value has been deemed to be reasonable for identifying individuals at high risk for future diabetes and to whom the term pre-diabetes may be applied.8 Renal insufficiency was defined as a pre-procedural creatinine level ≥1.5 mg/dL. Procedural success was defined as <50% residual diameter stenosis and Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow in the absence of major in-hospital complications (death, reinfarction, or urgent coronary artery bypass surgery). Acute myocardial infarction (AMI) was defined as the presence of new Q waves on a follow-up electrocardiogram or an elevation of creatinine kinase to ≥3 times normal. Cardiovascular mortality was classified as death attributable to MI, congestive heart failure, or arrhythmia.
The SPSS 20.0 (IBM Corp., Armonk, NY, USA) statistics program was used for all analyses. For discrete variables, differences between the two groups are expressed as counts and percentages, and were analyzed with Χ2 or Fisher's exact test. For continuous variables, differences between the two groups were evaluated by unpaired t test or Mann-Whitney rank test. Data are expressed as mean±SD. Statistical significance was defined as a p value of 0.05. The Cox proportional hazards regression model was used to estimate the independent association between glucose levels and long-term mortality rates.
The baseline clinical characteristics of the patients with prediabetes (group I) and the patients with normoglycemia (group II) are presented in Table 1. There was no significant difference in baseline characteristics between the two groups, except hypertension and prior PCI history which were higher in the prediabetic patients. Compared with normoglycemic patients, prediabetic patients tended to be older and to more commonly report prior percutaneous revascularization. As seen in Table 2, mean glucose levels were 104.8±51.27 mg/dL for patients with normoglycemia and 131.0±47.22 mg/dL for prediabetic patients (p<0.001). While patients with prediabetes showed higher total cholesterol level, low density lipoprotein-cholesterol did not differ with that in normoglycemia patients. The characteristics of coronary angiography are presented in Table 3. The reference vessel diameters (2.92±0.47 mm vs. 2.98±0.51 mm,p=0.123) and minimal luminal diameter (0.65±0.39 mm vs.0.68±0.41 mm, p=0.397) of the coronary artery were similar between the two groups. No significant differences were observed between the two groups with respect to other coronary angio-graphic parameters, except diffuse long lesion was more frequent in prediabetic patients than in normoglycemic patients (39.6% vs. 31.0%, p=0.024). Among the procedural characteristics, there were no significant differences in stent type and number and angiographic success rate between the two groups.
Six to 9-month angiographic outcomes and major clinical outcome at 2 years are displayed in Tables 4 and 5. In prediabetic patients, we noted trends toward a higher incidence of binary restenosis (15.6% vs. 9.8%, p=0.066) and late loss (0.71±0.70 mm vs. 0.59±0.62 mm, p=0.076). During the 24 months of follow up, mortality rate was significantly higher in the prediabetes group than the normoglycemia group (5.5% vs.1.5%, p=0.007). However, the incidences of cardiac death, MI, target lesion revascularization, and TVR were similar between the two groups. In analysis of independent predictors for mortality, prediabetes, age, chronic kidney disease (CKD), and chronic total occlusion (CTO) were identified as independent predictors of 24-month mortality (Table 6).
The present study determined that the presence of prediabetes in patients undergoing elective PCI with DESs is associated with worse mid-term angiographic outcomes and 2-year major clinical outcomes than those in patients with normoglycemia.
All stages of glucose abnormalities are associated with an increased risk of cardiovascular morbidity and mortality, making it important to identify them as early as possible.9 A significant proportion of dysglycemic individuals develop vascular damage during the pre-diabetes stage, although their glucometabolic perturbations often remain undetected until the first cardiovascular event.9 Prediabetes is associated with more advanced coronary artery disease, such as small vessel and diffuse coronary stenosis, compared with normoglycemia.10 The prevalence of patients with prediabetes and coronary artery disease varies from study to study. A previous study reported a high prevalence of abnormal glucose tolerance in 181 patients with AMI: IGT as prediabetes comprised 35% and diabetes 31%.11 In other studies, the prevalence of prediabetes and diabetes, respectively, were about 25% and 53% in patients who underwent coronary angiography.1012 Considering outcomes after PCI, Kataoka, et al.7 reported rates of MACE and repeat revascularization of 21% and 19% in a sirolimus-eluting stent group and 23% and 22% in a paclitaxel-eluting stent group, respectively, and the overall rate of non-fatal MI was about 5%. However, these results only reflected patients with 1st generation DES. There are no data on clinical and angiographic outcomes for second generation DES in prediabetes patients. Although it is hard to compare our results with above-mentioned study directly, our results, reflecting 1st and 2nd generation DESs, showed better results, compared to previous studies, demonstrating improved current clinical situations.
Some of the discrepancy with former studies might be explained as follows: First, HbA1c cutoff values differed among studies. Cut-off values for non-diabetics in previous studies largely comprised <6%, 6.7%, >7%, or fourth quartile. However, patients with HbA1c ≥6.5% were excluded in our study for more exact assessment of a prediabetic state. Applying an HbA1c cut-off value ≥6.5%, as suggested by the American Diabetes Association, in previous studies, non-diabetic patients were changed as DM on admission. More recent, stricter applications of guidelines might have affected the differences in clinical outcomes. Second, most previous studies demonstrated an association between HbA1c and MACE after AMI. Our results demonstrated clinical and angiographic outcomes for patients who underwent elective PCI, exhibiting a stable disease state. Finally, we included patients with 2nd generation DESs in reflection of current clinical practice. Indeed, the everolimus-eluting stent has been shown to be associated with significantly lower rates of adverse events, compared with first generation sirolimus-eluting stents, in all-comer DM patients.13
Our study revealed trends toward higher incidences of binary restenosis and late loss at 6.9-month angiographic follow up in comparison with normoglycemic patients. This result is consistent with studies in patients with diabetes. This trend of higher restenosis might have translated into higher incidences of TVR-MACE and total MACEs.
HbA1c values reflect a 2 to 3month average endogenous exposure to glucose, including postprandial spikes in blood glucose levels, and have low intra-individual variability, particularly in persons without diabetes.14 HbA1c values exceeding 6.0% may be a clinically useful marker to identify persons at risk for the development of not only diabetes but also cardiovascular disease and death.15 In studies using HbA1ccorresponding to prediabetes, an abnormal HbA1c level was deemed a significant independent predictor of MACE, TVR, and cardiovascular mortality at 12 months after PCI in non-diabetic patients.561617 Data from the Framingham study have indicated that, even in non-diabetic patients, there is an association between HbA1c and risk of cardiovascular disease.18 Kowalska, et al.19 demonstrated in a cohort of non-diabetic men referred for coronary angiography that the number of diseased vessels were significantly correlated with increasing levels of HbA1c. In our study, prediabetic patients were associated with higher trends of TVR MACE and all MACE and significantly higher mortality, compared to normoglycemic patients at 24 months. Further, the presence of prediabetes, as well as older age, CKD, and CTO lesion, was an independent predictor of mortality at 24 months (Table 6).
Our study has several limitations. First, this was a non-randomized, single-center retrospective analysis. This might have introduced significant bias in patient and device selection. Larger, multi-center studies would reinforce our results. Second, our selection process may have limited our findings, as we did not evaluate glucose metabolic function with an OGTT, which is necessary for accurate diagnosis of IGT.20 To diagnose prediabetes, our study protocol used HbA1c. No routine OGTT tests were performed to detect undiagnosed DM or prediabetes after admission, and thus, part of the observed association between hyperglycemia and outcomes might be due to undiagnosed DM or prediabetes in some patients. However, the International Expert Committee Report recommended using HbA1c assay as the preferred method for DM diagnosis, and suggested the diagnosis if HbA1c levels are ≥6.5%.21 The American Diabetes Association defines prediabetes as an HbA1c of 5.7% to 6.4%.8 HbA1c gives a better measure of overall glycemic exposure and likely risk for long-term complications. There is no need for fasting or timed samples, and HbA1c is less affected by conditions to produce perturbations in glucose levels. Moreover, it is a better guide for clinical management of patients. Third, in this study, routine angiographic follow-up was not performed, and thus, absolute restenosis rates of all of the study population could not be analyzed.
In conclusion, patients with prediabetes were found to be associated with higher trends of mid-term angiographic restenosis and late loss in our study. Further, there was a death rate among patients with prediabetes up to 2 years, compared to normoglycemic individuals, undergoing elective PCI with contemporary DESs. Even in the DES era, prediabetes using HbA1c can be a significant prognostic factor in patients undergoing elective PCI.
Figures and Tables
Table 1
Baseline Clinical Characteristics of the Study Population

Table 2
Laboratory Findings in the Study Population
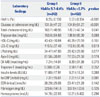
Table 3
Coronary Angiographic Characteristics
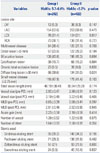
Table 4
Angiographic Outcomes at 6 to 9 Months

Table 5
Major Clinical Outcomes at 2 Years

Table 6
Predictors of Mortality at 24 Months

References
2. Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002; 19:708–723.

3. DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011; 108:3 Suppl. 3B–24B.

4. DECODE Study Group. the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001; 161:397–405.
5. Corpus RA, O'Neill WW, Dixon SR, Timmis GC, Devlin WH. Relation of hemoglobin A1c to rate of major adverse cardiac events in nondiabetic patients undergoing percutaneous coronary revascularization. Am J Cardiol. 2003; 92:1282–1286.

6. Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011; 124:704–711.

7. Kataoka Y, Yagi N, Kokubu N, Kasahara Y, Abe M, Otsuka Y. Efficacy of paclitaxel-eluting stent in patients with impaired glucose tolerance--comparison with sirolimus-eluting stent. Circ J. 2011; 75:868–873.

8. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33:Suppl 1. S62–S69.
9. Anselmino M, Wallander M, Norhammar A, Mellbin L, Rydén L. Implications of abnormal glucose metabolism in patients with coronary artery disease. Diab Vasc Dis Res. 2008; 5:285–290.

10. Kataoka Y, Yasuda S, Morii I, Otsuka Y, Kawamura A, Miyazaki S. Quantitative coronary angiographic studies of patients with angina pectoris and impaired glucose tolerance. Diabetes Care. 2005; 28:2217–2222.

11. Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendíc S, Rydén L, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002; 359:2140–2144.

12. de la Hera JM, Delgado E, Hernández E, García-Ruiz JM, Vegas JM, Avanzas P, et al. Prevalence and outcome of newly detected diabetes in patients who undergo percutaneous coronary intervention. Eur Heart J. 2009; 30:2614–2621.

13. Kedhi E, Gomes ME, Lagerqvist B, Smith JG, Omerovic E, James S, et al. Clinical impact of second-generation everolimus-eluting stent compared with first-generation drug-eluting stents in diabetes mellitus patients: insights from a nationwide coronary intervention register. JACC Cardiovasc Interv. 2012; 5:1141–1149.

14. Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007; 167:1545–1551.

15. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010; 362:800–811.

16. Timmer JR, van der Horst IC, Ottervanger JP, Henriques JP, Hoorntje JC, de Boer MJ, et al. Prognostic value of admission glucose in non-diabetic patients with myocardial infarction. Am Heart J. 2004; 148:399–404.

17. Lee YS, Jeong MH, Kim KH, Kang DG, Yun KH, Lee SH, et al. The relationship between hemoglobin A1c and major adverse cardiac events in nondiabetic acute myocardial infarction patients underwent primary percutaneous coronary intervention. Korean Circ J. 2005; 35:369–374.

18. Singer DE, Nathan DM, Anderson KM, Wilson PW, Evans JC. Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Diabetes. 1992; 41:202–208.

19. Kowalska I, Prokop J, Bachórzewska-Gajewska H, Telejko B, Kinalskal I, Kochman W, et al. Disturbances of glucose metabolism in men referred for coronary arteriography. Postload glycemia as predictor for coronary atherosclerosis. Diabetes Care. 2001; 24:897–901.

20. Farhan S, Jarai R, Tentzeris I, Kautzky-Willer A, Samaha E, Smetana P, et al. Comparison of HbA1c and oral glucose tolerance test for diagnosis of diabetes in patients with coronary artery disease. Clin Res Cardiol. 2012; 101:625–630.

21. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009; 32:1327–1334.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download