Abstract
Purpose
Loss of cholinergic neurons in the hippocampus is a hallmark of many dementias. Administration of stem cells as a therapeutic intervention for patients is under active investigation, but the optimal stem cell type and transplantation modality has not yet been established. In this study, we studied the therapeutic effects of human placenta-derived mesenchymal stem cells (pMSCs) in dementia rat model using either intracerebroventricular (ICV) or intravenous (IV) injections and analyzed their mechanisms of therapeutic action.
Materials and Methods
Dementia modeling was established by intraventricular injection of 192 IgG-saporin, which causes lesion of cholinergic neurons. Sixty-five male Sprague-Dawley rats were divided into five groups: control, lesion, lesion+ICV injection of pMSCs, lesion+IV injection of pMSCs, and lesion+donepezil. Rats were subjected to the Morris water maze and subsequent immunostaining analyses.
Results
Both ICV and IV pMSC administrations allowed significant cognitive recovery compared to the lesioned rats. Acetylcholinesterase activity was significantly rescued in the hippocampus of rats injected with pMSCs post-lesion. Choline acetyltransferase did not co-localize with pMSCs, showing that pMSCs did not directly differentiate into cholinergic cells. Number of microglial cells increased in lesioned rats and significantly decreased back to normal levels with pMSC injection.
Conclusion
Our results suggest that ICV and IV injections of pMSCs facilitate the recovery of cholinergic neuronal populations and cognitive behavior. This recovery likely occurs through paracrine effects that resemble microglia function rather than direct differentiation of injected pMSCs into cholinergic neurons.
Dementia is a neurodegenerative disease of the brain, resulting in impairment of mental abilities. Many cases of dementia, including Alzheimer's disease (AD), are characterized by denervation of cholinergic neurons in the hippocampus and entorhinal cortex and subsequent memory impairment.1 While the exact pathophysiology of dementia is still under active debate, studies have shown reduction in choline acetyltransferase (ChAT) activity in the hippocampus and frontal cortex of dementia patients.2 Additionally, lesions of basal forebrain cholinergic neurons in rats induced by the injection of 192 IgG-saporin, an immunotoxin, leads to cholinergic hypofunction and eventually results in considerable impairment of cognitive function and behavior.3 Thus, various previous studies have utilized 192 IgG-saporin administrations as a method of neurodegenerative dementia rat model establishment.456
Acetylcholinesterase (AChE) inhibitors are an FDA-approved drug for dementia patients, but this method has shown limited efficacy, calling for a more effective treatment method. Alternatively, mesenchymal stem cells (MSCs) have been actively researched for their therapeutic potential against neurodegenerative disorders,7 and MSCs were found to secrete neuroregulatory factors that carry out neuroprotective actions and promote neurogenesis.8 Some studies using MSC have reported promising results for neurodegenerative disease models. In 6-hydroxydopamine-induced Parkinson's disease (PD) rat models, intravenous administration of human bone marrow-derived MSCs was found to alleviate explicit parkinsonian symptoms, suggesting that MSC administration could elicit therapeutic effects on neurodegenerative diseases pertaining to dopaminergic neurons.9 Also, human adipose tissue-derived MSCs (ADSCs) facilitated β-amyloid peptide (Aβ) clearance via in vitro secretion of exosomes containing the Aβ-degrading enzyme neprilysin, suggesting a possible therapeutic approach to AD using ADSCs.10
While MSC administration for therapeutic intervention of neurodegenerative disorders is rising in popularity, limited information is available regarding the most appropriate source of MSCs for transplantation. Human placenta-derived MSCs (pMSCs) exhibit high adhesive and secretory properties.11 Moreover, procurement of pMSCs does not conflict with ethical concerns12 and they display phenotypic plasticity.13 They are also immunologically privileged14 and are easily obtainable without invasive procedures. Based on such advantages, pMSCs seem to be a viable candidate for transplantation in neurodegenerative dementia models.
MSC transplantation was shown to influence microglia activation in AD mouse models and reduce Aβ deposition.15 Microglia are glial cells that defend the neural parenchyma from factors such as infections, ischemia, and neurodegeneration.15 Microglial cells are known to help maintain neural stem cell homeostasis and participate in immunological actions through phagocytic activity.16 In addition, microglia have been shown to serve neuroprotective functions through secretion of various factors and increasing neuronal survival,17 and microglia in the hippocampus of stroke rat models were shown to protect neurons by synthesizing tumor necrosis factors.18 Thus, we anticipated that active microglia quantity would grow in dementia model rats with lesions of cholinergic neurons and decrease upon appropriate treatments that allow recovery in cholinergic function.
As mentioned above, there have been studies showing that stem cell administration alleviates symptoms in PD and AD models. However, to our knowledge, the therapeutic effect of pMSCs in dementia models with cholinergic neuronal degeneration and its associated mechanisms are less understood. In this study, we evaluated the behavioral recovery effects of pMSC administration in 192 IgG-saporin-induced dementia model rats in comparison with the current standard treatment using donepezil, an AChE inhibitor.
Furthermore, we investigated the therapeutic effects of administration of pMSCs in dementia model rats using two different cell injection methods, intracerebroventricular (ICV) and intravenous (IV) injections, and also the possible mechanisms of therapeutic action. Hemodynamic considerations may suggest better stem cell delivery through intra-arterial (IA) injections, however, our previous IA injection trials entailed several difficulties: considerable invasiveness involving incision through neck skin and muscles, uncontrollable bleeding, and extended recovery time post-operation. Thus, as the two prime injection methods of our study, we selected the ICV route, which is invasive but highly target-specific, and the IV route, which allows marginal invasiveness and faster stem cell administration.
All animal experiments in this study were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Yonsei University, Korea.
Procedures were carried out according to the experiment timeline (Fig. 1). Rats were housed in groups of three per cage with food and water ad libitum, and they were kept in a temperature/humidity-controlled room with a 12-hour light/12-hour dark cycle. Efforts were taken to minimize the number of rats used and overall animal suffering.
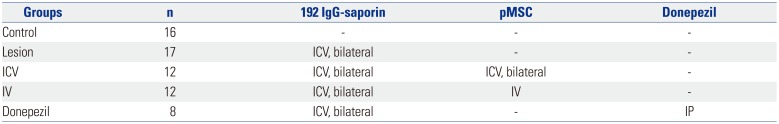
Experimental grouping is summarized in Table 1. Sixty-five male Sprague-Dawley rats (180–200 g) were randomly assorted into five groups before surgery. The control group rats (n=16) did not undergo any surgical procedures. The lesion group rats (n=17) received bilateral ventricular infusion of 192 IgG-saporin into the brain. Rats in the ICV transplantation group (n=12) were bilaterally infused with 192 IgG-saporin in the lateral ventricle and received ICV injection of pMSCs one week later. Rats in the IV transplantation group (n=12) were bilaterally infused with 192 IgG-saporin in the lateral ventricle and received tail vein injection of pMSCs one week afterwards. Lastly, rats in the donepezil group (n=8) were bilaterally infused with 192 IgG-saporin in the lateral ventricle and were intraperitoneally injected with donepezil every day after pMSC transplantation in the ICV and IV group rats.
Forty-nine rats (excluding the control group) were anesthetized with a mixture of ketamine (75 mg/kg), xylazine (Rompun™ 4 mg/kg; Bayer Korea, Seoul, Korea), and acepromazine (0.75 mg/kg) and were secured in a stereotaxic frame. Scalp skin was incised, and two holes were drilled into the skull [anteroposterior (AP) −0.8 mm, mediolateral (ML) ±1.2 mm, dorsoventricular (DV) −3.4 mm]. Afterwards, 8 µL of 192 IgG-saporin (0.63 µg/µL; Chemicon, Temecula, CA, USA) were bilaterally injected into the lateral ventricle at a rate of 1 µL/min and was left to diffuse for 5 min after injection.
pMSCs were harvested and isolated from normal human placenta according to protocols described in previous studies.19 MSC harvestation procedure on human donors was approved by the Institutional Review Board of CHA General Hospital, Seoul, Korea. Cell surface marker profiling in our previous studies also revealed that the pMSCs were negative for CD34 (a hematopoietic and endothelial cell marker), SSEA4, TRA-1-60, and TRA-1-81 (embryonic stem cell markers), and positive for CD9 (a nontrophoblast marker), CD13 and CD90 (MSC markers), and CD200 (an immunoregulator).19 One week after 192 IgG-saporin injections, 12 rats in the ICV group were anesthetized with a mixture of ketamine, xylazine, and acepromazine. Afterwards, the rats received 6 µL of pMSC (9×105 cells/6 µL, 3 µL/5 min) transplantation into the following coordinates: AP −0.8 mm, ML ±1.2 mm, DV −3.4 mm. The pMSCs were allowed to diffuse for 5 min after each injection. Twelve rats in the IV group received 200 µL of pMSCs (5×106 cells/200 µL) via tail vein injection. All rats were immunosuppressed with cyclosporine [12.5 mg/kg, daily intraperitoneal (IP) injection] starting the day before transplantation for up to five weeks. Eight rats were used for the donepezil injection group (3 mg/kg, daily IP injection).
Additionally, in order to exclude the possible effects of surgical procedures on microglial cell count, four rats were sham-operated [bilateral injection of 8 µL phosphate buffer saline (PBS)], and their microglial cell counts were compared with those of control group rats.
Five weeks after pMSC transplantation, all rats underwent the Morris water maze test. The Morris water maze apparatus was made up of a circular pool 2 m in diameter and 0.5 m in depth. This pool was filled with opaque tap water (23℃) and concealed a circular (0.15 m in diameter) black escape platform submerged 2 cm below the surface of the water. All rats were trained for four trials per day. These four trials continued for 5 consecutive days with a fixed hidden platform guided by spatial cues. For each training trial, the rat was semi-randomly placed into one of the four start points and was given 60 sec to reach the hidden platform. After finding the platform, the rat was allowed to remain on the platform for 10 sec. Rats that failed to reach the platform within 60 sec were led to the platform by the experimenter and were allowed to remain on the platform for 10 sec. Twenty-four hours after the final training trial, the rats were given probe tests. Each probe test lasted for 60 sec without the platform. Swimming distance, time spent in each zone, swim path, and speed were recorded and computed by the SMART video-tracking system (Harvard Apparatus, Holliston, MA, USA).
After the behavioral tests, 8 out of 16 rats from the control group, 8 out of 17 rats from the lesion group, 6 out of 12 rats from the ICV group, 6 out of 12 rats from the IV group, and 4 out of 8 rats from the donepezil injection group were anesthetized with a mixture of ketamine (75 mg/kg), xylazine (4 mg/kg), and acepromazine (0.75 mg/kg) and underwent transcardial perfusion with normal saline and cold 4% paraformaldehyde. Brains were removed, post-fixed, transferred to 30% sucrose, and stored for 3 days. The brains were sectioned into 35 µm slices using a freezing microtome and stored in a cryoprotectant solution [0.1 M phosphate buffer (pH 7.2), 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol] at −20℃.
The remaining rats from each group were anesthetized with a mixture of ketamine (75 mg/kg), xylazine (4 mg/kg), and acepromazine (0.75 mg/kg) and then decapitated with a guillotine. The brains were quickly removed. The prefrontal cortex, hippocampus, and medial septum (MS) regions were dissected with fine forceps from 1 mm coronal brain slices. These samples were homogenized in lysis buffer (Intron, Seongnam, Korea) and centrifuged for 10 min at 12000 rpm. The protein in the supernatant was measured using the bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL, USA). The protein samples were stored at −70℃.
To evaluate the enzymatic activity of AChE, the method described by Ellman, et al.20 was used. In brief, 20 µL triplicate samples were mixed with a reaction mixture [0.2 mM dithiobisnitrobenzoic acid (Sigma-Aldrich, Louis, MO, USA), 0.56 mM acetylthiocholine iodide (Sigma-Aldrich), 10 µM tetraisopropyl pyrophosphoramide (Sigma-Aldrich), and 39 mM phosphate buffer; pH 7.2] at 37℃. After 30 min, the optical density was measured at 405 nm.
Diaminobenzidine (DAB) staining was performed to evaluate the location and number of pMSCs. For immunohistochemistry, brain sections were incubated in 0.3% H2O2 for 60 min to block endogenous peroxidase activity. The sections were blocked with 5% normal goat serum and incubated overnight with monoclonal antibodies against a human cytoplasmic protein (STEM121, AB-121-U-050, 1:300; StemCells, Cambridge, UK) at 4℃. Afterwards, the sections were incubated with antimouse biotinylated IgG secondary antibodies (BA-9200; Vector Laboratories, Burlingame, CA, USA), followed by the avidin-biotin complex method (ABC Elite; Vector Laboratories). The cells were then visualized using a DAB substrate kit (Thermo, Fremont, CA, USA). The samples were examined using a virtual microscope (BX51; Olympus, Tokyo, Japan).
For immunofluorescence analysis of ChAT, sections were incubated overnight at 4℃ with monoclonal antibodies against human nuclei (MAB1281; Merck Millipore, Billerica, MA, USA) and ChAT (Anti-ChAT, 1:100; Merck Millipore). The sections were then incubated with anti-mouse Cy5 (A10524, 1:500; Life Technologies, Carlsbad, CA, USA) and anti-goat FITC (Ab6881, 1:200; Santa Cruz Biotechnology, Dallas, TX, USA). For immunofluorescence analysis of microglial cells, sections were incubated overnight at 4℃ with monoclonal antibodies against microglia (Iba1, 019-19741, 1:400; Wako Chemicals, Richmond, VA, USA). The sections were subsequently incubated with anti-rabbit Alexa Fluor 488 (A11008, 1:500; Invitrogen, Carlsbad, CA, USA) secondary antibodies. The samples were examined and photographed with a confocal laser scanning microscope (LSM700; Carl Zeiss, Jena, Germany).
Statistical analyses were conducted using SPSS software ver. 21 (IBM Corp., Armonk, NY, USA). All analyses were performed using one-way analysis of variance (ANOVA) followed by post hoc Fisher's least significant difference method for exploratory analysis of our factors. Data are presented as mean±standard error. p-value <0.05 was considered statistically significant.
Prior to probe testing, all rats were trained in the water maze for four trials per day for five consecutive days. On average, rats in all five groups showed a gradual decline in latency to the platform over the five days (Fig. 2A). Twenty-four hours after the final training trial, the rats were subjected to probe testing. Three measurements were made in the probe trial: time spent in the target quadrant, time spent in the platform zone, and the number of platform zone crossings.
In the probe test, the lesion group rats showed poor performance compared to the control rats in all three measurements of interest (Fig. 2B, C, and D). ICV and IV group rats all showed significant improvement in all three measurements compared to the lesioned rats (Fig. 2B, C, and D). Donepezil group rats also showed significant improvement in time spent in the target quadrant and in the platform area but did not show a significant difference from the lesion group in the number of platform crossings (Fig. 2B, C, and D).
In order to compare cholinergic neuronal activity, we quantified AChE activity in all groups using Ellman's methods.20 This measurement was made in three parts of the rats' brains: the medial prefrontal cortex (mPFC), the hippocampus, and the MS. In the mPFC and hippocampal regions, AChE activity was significantly reduced in the lesion group compared to the control group (Fig. 3A and B). AChE activity in the MS also declined in the lesion group, although the difference from controls was not statistically significant (Fig. 3C). In the mPFC and hippocampus, AChE activities of ICV and IV group rats significantly improved compared to that of the lesioned rats, suggesting that pMSC administration boosts cholinergic neuronal function to a level similar to rats without dementia (Fig. 3A and B). In contrast, lesion, ICV, IV, and donepezil group rats all tended to show lower levels of AChE activity in the MS region compared to control rats, but the difference was not significant (Fig. 3C). Overall, ICV and IV pMSC administrations showed therapeutic effects similar to that of donepezil.
Placental MSC homing was investigated for ICV and IV groups to evaluate the effectiveness of the injection methods. The injected pMSCs were counted after DAB staining for STEM121, and indeed certain portions of pMSCs were found for both methods (ICV 2727.5±574.332 cells; IV 7172.5±1041.075 cells) at the dentate gyrus of the hippocampal region (Fig. 4). Percentages of localized pMSCs at the dentate gyrus with respect to the total number of injected cells were approximately 0.30% in the ICV group and 0.14% in the IV group.
To take into account the possibility of differentiation of the injected stem cells into cholinergic neurons, rat brain sections from all groups were subjected to immunofluorescence analysis using markers for human nuclei and for ChAT. Many stem cells were found in the hippocampus, but we could not detect any co-localizations between the stem cell nuclei marker and the ChAT marker (Fig. 5). The injected stem cells did not seem to differentiate into cholinergic neurons.
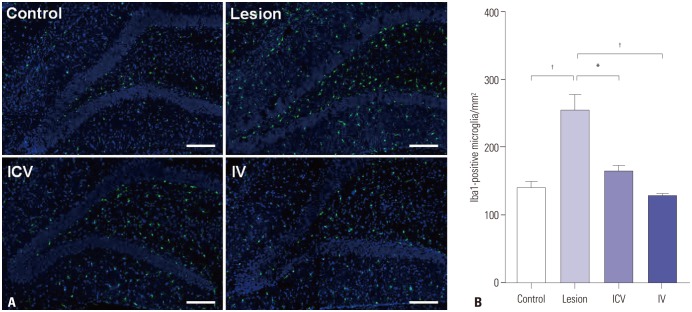
Microglial cell count was carried out for control, lesion, ICV, and IV group rats using the microglial cell marker, Iba1. The cell count was performed for the dentate gyrus region. In order to exclude the possibility of any influences on microglial cell count invoked by the operation procedures, numbers of microglial cells were compared across the four groups together with a fifth sham-operated group. One-way ANOVA results showed similar trends in microglial cell count for the control and the sham-operated groups: both groups showed a significant difference from the lesion group, whereas both groups showed no significant difference from either ICV or IV groups (significance level of 0.05 for all pairwise comparisons). The normal and sham-operated groups did not show a significant difference from each other. For consistency, data for sham-operated group rats were not included in the figure. The number of microglial cells markedly increased; the control group (326.7±22.11 cells) and the lesion group (541.4±19.55 cells). Both ICV and IV administration of pMSCs led to a significant decrease of microglia count compared to the lesion group (ICV 382.6±10.43 cells; IV 282.8±11.35 cells) (Fig. 6). Both ICV and IV groups showed no significant difference from the control group in microglial cell count.
Dementia caused by cholinergic neuronal degeneration is currently treated with AChE inhibitors such as donepezil, but these drugs have limited efficacy. Stem cell therapy is now recognized as an alternative therapeutic tool for neurodegenerative diseases for its potential in differentiation and paracrine secretions. The efficiencies of different MSC transplant modalities and relevant mechanisms, however, are not yet clarified.
All rats showed improvement in the Morris water maze training phase, indicating that all rats managed to adopt a certain strategy for escaping the water. It was reported that rats with lesions of cholinergic pathways show poor acquisition of spatial memory and that they alternatively adopt a non-spatial search strategy.21 Our water maze results were in accordance with such findings; lesioned rats still showed similar improvement in training latency to the control rats. It is, therefore, highly likely that the lesioned rats used a random search strategy to find the platform rather than relying on learning and spatial cognitive functions; this strategy is also apparent in the water maze probe test results in which the lesioned rats displayed significantly poorer performance than the control rats in all three measurements (Fig. 2). This speculation is also consistent with former findings which showed that cholinergic dysfunction leads to downregulation of memory-related proteins, resulting in cognitive impairment and memory loss.22 Also, in the probe test, ICV, IV, and donepezil group rats all recovered in performance compared to lesioned rats (except for the donepezil group in platform crossings), suggesting that pMSC administration facilitated effects comparable to donepezil. Additionally, we did not observe significant differences in terms of behavioral improvement between ICV and IV groups. Thus, we conclude that neither stem cell administration pathway is superior over the other in regard with the effect on cognitive behavior.
Since the MS-hippocampus-mPFC memory circuit consists of a cholinergic system,1221 we first assessed cholinergic neuronal activities in the three regions of interest using an AChE assay. Injection of 192 IgG-saporin tended decrease AChE activity in the MS and led to a decline in AChE activities in the downstream hippocampus and mPFC regions as well (Fig. 3). Stem cell administration through ICV and IV routes resulted in no significant AChE activity increase from the lesion group in the MS region, but led to AChE functional recovery in the hippocampus (Fig. 3B). Since the dentate gyrus of the hippocampus is a prime source of granule cell neurogenesis23 and the neighboring periventricular area also exhibits neural stem cells,24 the hippocampal region provides an adequate environment for stem cell homing and neurogenesis. In effect, the injected pMSCs possibly migrated to the hippocampal region and helped restore cholinergic activity in the hippocampus, increasing cholinergic innervation to the mPFC and enhancing cholinergic activity at the mPFC as well. It is quite likely that the MS did not improve cholinergic activity due to its placement upstream of the hippocampus in the circuit.
In order to verify whether the injected stem cells actually reached the hippocampal region, brain tissue sections of the rats in ICV and IV groups were stained using DAB immunohistochemistry for human cytoplasm. Stem cells were found localized in the hippocampus for both groups, mainly at the dentate gyrus (Fig. 4). Both IV and ICV administration of pMSCs into rodents allowed migration of the stem cells to the malfunctioning site of the brain parenchyma, which is consistent with earlier findings.252627 Although earlier studies found that most MSCs are filtered in the lungs, kidneys, spleen, and liver upon IV injection and only a small fraction of MSCs reach the brain target,28 our study showed that IV injection nonetheless resulted in some pMSC localization at the hippocampus and subsequent recovery in cognitive functions. Considering its minor invasiveness and easier administration, IV injection may also be relatively advantageous compared to ICV injection of pMSCs. However, in terms of cholinergic cell rescue, neither ICV nor IV injection method displayed significant dominance over one another: both methods yielded similar results.
Our main question was how these transplanted stem cells helped restore cholinergic activity in the hippocampus. Our first hypothesis was that the injected pMSCs, once reaching the hippocampus, differentiated into cholinergic cells and replaced the lesions caused by injection of 192 IgG-saporin. However, ChAT immunostaining results showed no co-localization between human nuclei and ChAT markers (Fig. 5), indicating that this was not the case. Such results are also consistent with earlier studies. MSC administration is thought to regulate Aβ accumulation not through direct differentiation but through interactions with local immune cells,29 and an in vitro experiment using a cellular model of AD showed that MSC and MSC secretome prolongs cell viability and promotes neuritogenesis, suggesting that MSC secretome has therapeutic potentials for neurodegenerative diseases.30 The facts that a certain portion of our injected pMSCs reached the hippocampus of the rats (Fig. 4), and that these pMSCs did not differentiate into cholinergic neurons (Fig. 5), also suggest that there is a more complex therapeutic mechanism of injected pMSCs than mere differentiation.
Since many previous studies reported that immune reactions are triggered by implanted MSCs in the brain,2931 we investigated participants of immune reactions in the brain, microglia in our case, that may have influenced behavioral rehabilitation of dementia model rats after MSC administration. As mentioned in the results, lesioned rats showed significantly increased numbers of microglial cells at the dentate gyrus of the hippocampus, and rats of both ICV and IV groups displayed significantly reduced numbers of microglia compared to the lesioned rats and showed no significant difference from control rats (Fig. 6B). A limitation of study might be that normal rats were used as the control instead of sham-operated rats for experiments. However, statistical analysis of microglial cell counts revealed a similar trend between normal and sham-operated rats, as shown in the results section.
Microglia are glial cells in the central nervous system that participate in innate immunity in response to pathology through numerous mechanisms, such as physical contact with and removal of injured neurons and their synapses,32 or even phagocytosis of whole neurons.33 This fundamental role of phagocytosis is essential for facilitation of neuronal circuit reorganization, anti-inflammatory responses, and regeneration of new neurons.34 However, excessive phagocytosis activates production of toxic reactive oxygen species that can be detrimental to the surrounding neurons as well.35 The phagocytic activity of microglial cells can also lead to neuronal loss due to phagocytosis of live neurons and live neural precursor cells.36 These results suggest that even though microglia carry out the beneficial function of clearing out damaged neuronal debris, excessively large numbers of microglial cells may not be desirable for normal neurons.
Therefore, our results showing that IV and ICV injections of pMSCs lower microglia count to normal levels imply that pMSCs serve a dual function of neural recovery and neuroprotection. Again, no significant difference in microglial counts was observed between IV and ICV groups, suggesting that neither method was superior over the other. Administration of pMSCs facilitated recovery of cognitive rehabilitation (Fig. 2) and cholinergic neuronal function in dementia model rats (Fig. 3), while keeping the number of microglial cells in check, protecting functional neurons from unnecessary phagocytosis (Fig. 6). Although the mechanism by which these phenomena take place remains as a subject for further research, it is possible that the injected pMSCs take over the neural repair roles of microglia. Besides phagocytosis, microglia promotes generation of new neurons through secretion of signaling molecules such as brain-derived neurotrophic factor (BDNF).373839 Also, although still under debate, some studies have reported that human MSC transplantation into rats leads to an increase in BDNF levels at the injury site.40 Perhaps, similar paracrine effects of pMSCs facilitate the recovery of cholinergic neuronal systems, consequently leading to cognitive restoration of dementia model rats.
In summary, our results demonstrated that administration of pMSCs into dementia model rats facilitated recovery of cholinergic activity and cognitive function. This is supported by the results of the AChE assay and Morris water maze test. In addition, we found that both IV and ICV administrations of pMSCs were effective for targeted delivery of the stem cells into the hippocampus of the dementia model rats, and that neither injection method was significantly more effective than the other. As previously discussed, both ICV and IV routes have their advantages and drawbacks: ICV administration is highly target-specific but invasive, whereas IV injection is less invasive but is subject to filtration of pMSCs in various organs. Thus, both methods are reasonable candidates for stem cell administration, and the choice of method should be made under careful consideration of physiological conditions of the subject. Moreover, our results showed that the injected pMSCs did not directly differentiate into cholinergic neurons, but rather influenced generation of cholinergic neurons via some other mechanism. It is highly possible that microglia are one of the main factors involved in this process, and that pMSCs exert functions similar to microglial cells, such as secretion of trophic factors. Although further researches are necessary for clarification of the exact mechanisms involved and establishment of optimal conditions for successful stem cell homing, the therapeutic effects of pMSCs, nonetheless, shed light on possible future clinical applications of stem cell therapy to dementia and other neurodegenerative diseases.
ACKNOWLEDGEMENTS
This study was supported by the Industrial Strategic Technology Development Program (10062880).
References
1. Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ. Choline acetyltransferase activity and cognitive domain scores of Alzheimer's patients. Neurobiol Aging. 2000; 21:11–17. PMID: 10794843.

2. Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain. 2014; 137:2493–2508. PMID: 25062696.

3. Ricceri L, Minghetti L, Moles A, Popoli P, Confaloni A, De Simone R, et al. Cognitive and neurological deficits induced by early and prolonged basal forebrain cholinergic hypofunction in rats. Exp Neurol. 2004; 189:162–172. PMID: 15296846.

4. Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 1995; 109:714–722. PMID: 7576215.

5. Gelfo F, Petrosini L, Graziano A, De Bartolo P, Burello L, Vitale E, et al. Cortical metabolic deficits in a rat model of cholinergic basal forebrain degeneration. Neurochem Res. 2013; 38:2114–2123. PMID: 23925861.

6. Lehmann O, Grottick AJ, Cassel JC, Higgins GA. A double dissociation between serial reaction time and radial maze performance in rats subjected to 192 IgG-saporin lesions of the nucleus basalis and/or the septal region. Eur J Neurosci. 2003; 18:651–666. PMID: 12911761.

7. Tanna T, Sachan V. Mesenchymal stem cells: potential in treatment of neurodegenerative diseases. Curr Stem Cell Res Ther. 2014; 9:513–521. PMID: 25248677.

8. Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration. Cell Mol Life Sci. 2013; 70:3871–3882. PMID: 23456256.

9. Suzuki S, Kawamata J, Iwahara N, Matsumura A, Hisahara S, Matsushita T, et al. Intravenous mesenchymal stem cell administration exhibits therapeutic effects against 6-hydroxydopamine-induced dopaminergic neurodegeneration and glial activation in rats. Neurosci Lett. 2015; 584:276–281. PMID: 25449872.

10. Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013; 3:1197. PMID: 23378928.

11. Zhu SF, Zhong ZN, Fu XF, Peng DX, Lu GH, Li WH, et al. Comparison of cell proliferation, apoptosis, cellular morphology and ultrastructure between human umbilical cord and placenta-derived mesenchymal stem cells. Neurosci Lett. 2013; 541:77–82. PMID: 23523648.

12. Parolini O, Soncini M. Placenta as a source of stem cells and as a key organ for fetomaternal tolerance. In : Bhattacharya N, Stubblefield P, editors. Regenerative medicine using pregnancy-specific biological substances. London: Springer London;2011. p. 11–23.
13. Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008; 26:300–311. PMID: 17975221.

14. Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010; 7:168–182. PMID: 21060975.

15. Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett. 2009; 450:136–141. PMID: 19084047.
16. Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013; 61:121–127. PMID: 22927325.

17. Boscia F, Esposito CL, Di Crisci A, de Franciscis V, Annunziato L, Cerchia L. GDNF selectively induces microglial activation and neuronal survival in CA1/CA3 hippocampal regions exposed to NMDA insult through Ret/ERK signalling. PLoS One. 2009; 4:e6486. PMID: 19649251.

18. Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009; 29:1319–1330. PMID: 19193879.

19. Kim KS, Kim HS, Park JM, Kim HW, Park Mk, Lee HS, et al. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging. 2013; 34:2408–2420. PMID: 23623603.

20. Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961; 7:88–95. PMID: 13726518.

21. Nilsson OG, Strecker RE, Daszuta A, Björklund A. Combined cholinergic and serotonergic denervation of the forebrain produces severe deficits in a spatial learning task in the rat. Brain Res. 1988; 453:235–246. PMID: 3401761.

22. Chen TJ, Chen SS, Wang DC, Hung HS. The cholinergic signaling responsible for the expression of a memory-related protein in primary rat cortical neurons. J Cell Physiol. 2016; 231:2428–2438. PMID: 26895748.

23. Tatebayashi Y, Lee MH, Li L, Iqbal K, Grundke-Iqbal I. The dentate gyrus neurogenesis: a therapeutic target for Alzheimer’s disease. Acta Neuropathol. 2003; 105:225–232. PMID: 12557008.

24. Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002; 22:1784–1793. PMID: 11880507.

25. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999; 96:10711–10716. PMID: 10485891.

26. Lee SH, Jin KS, Bang OY, Kim BJ, Park SJ, Lee NH, et al. Differential migration of mesenchymal stem cells to ischemic regions after middle cerebral artery occlusion in rats. PLoS One. 2015; 10:e0134920. PMID: 26241653.

27. Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001; 12:559–563. PMID: 11234763.

28. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009; 18:683–692. PMID: 19099374.

29. Hamisha KN, Tfilin M, Yanai J, Turgeman G. Mesenchymal stem cells can prevent alterations in behavior and neurogenesis induced by Aß25-35 administration. J Mol Neurosci. 2014; 55:1006–1013. PMID: 25384918.

30. Zilka N, Zilkova M, Kazmerova Z, Sarissky M, Cigankova V, Novak M. Mesenchymal stem cells rescue the Alzheimer’s disease cell model from cell death induced by misfolded truncated tau. Neuroscience. 2011; 193:330–337. PMID: 21763758.

31. Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY, et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol Dis. 2013; 58:249–257. PMID: 23759293.

32. Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013; 77:10–18. PMID: 23312512.

33. Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011; 186:4973–4983. PMID: 21402900.

34. Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009; 132(Pt 2):288–295. PMID: 18567623.

35. Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013; 7:6. PMID: 23386811.

36. Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013; 33:4216–4233. PMID: 23467340.

37. Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brainderived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999; 19:1708–1716. PMID: 10024357.

38. Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005; 438:1017–1021. PMID: 16355225.

39. Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S, Kurihara T. Neurotrophin secretion from cultured microglia. J Neurosci Res. 2001; 65:322–331. PMID: 11494368.

40. Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010; 1343:226–235. PMID: 20470759.

Fig. 2
Morris water maze test results. (A) All five groups showed a tendency to improve in cognitive performance over the five training sessions. (B, C, and D) Probe test results after training: time spent in the target quadrant (B), time spent in the platform zone (C), and the number of platform crossings (D). Comparisons among groups were made using one-way analysis of variance followed by post hoc Fisher's least significant difference method. *p<0.05, †p<0.01, ‡p<0.001 for comparisons. ICV, intracerebroventricular; IV, intravenous.
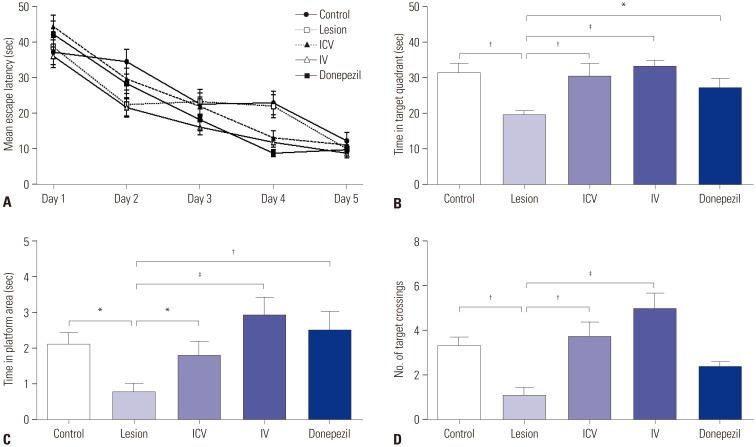
Fig. 3
AChE assay. Quantification of AChE was carried out for three brain regions involved in the memory circuit: the mPFC (A), the hippocampus (B), and the MS (C). Comparisons among groups were made using one-way analysis of variance followed by post hoc Fisher's least significant difference method. *p<0.05, †p<0.01 for comparisons. AChE, acetylcholinesterase; mPFC, medial prefrontal cortex; MS, medial septum; ICV, intracerebroventricular; IV, intravenous.
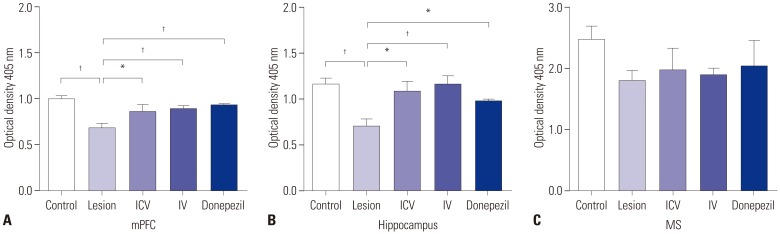
Fig. 4
DAB staining (×20, objective lense) for STEM121 human cytoplasmic marker. DAB staining was performed for investigation of stem cell homing at the dentate gyrus region after pMSC administration. The pMSCs were found at the dentate gyrus region after both ICV and IV injections. No pMSCs were found in the control and lesion groups. Scale bar=100 µm. DAB, diaminobenzidine; pMSC, placenta-derived mesenchymal stem cell; ICV, intracerebroventricular; IV, intravenous.
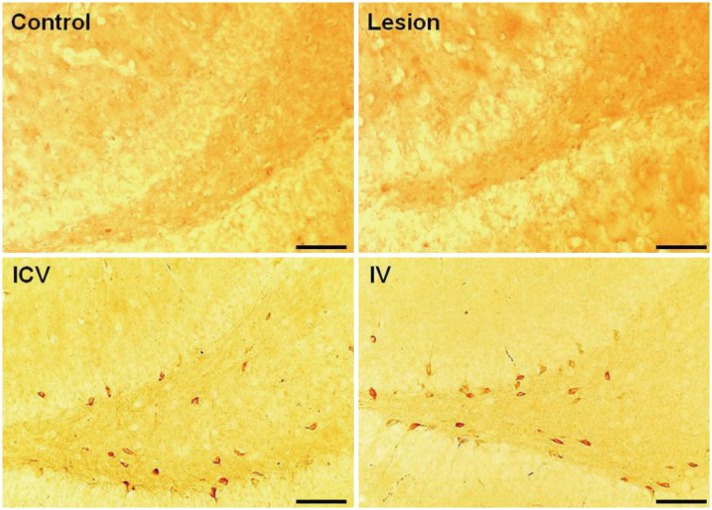
Fig. 5
Immunofluorescence analysis of ChAT activity at the dentate gyrus region. Samples were stained with hNu, anti-ChAT, and DAPI (×20, objective lense). White arrows indicate cholinergic neurons and transplanted pMSCs in ChAT and hNu images, respectively. No co-localization was found between hNu and ChAT markers (white arrows), indicating that the injected pMSCs did not directly differentiate into cholinergic cells. Scale bar=40 µm. hNu, human anti-nuclei marker; ChAT, choline acetyltransferase; pMSC, placenta-derived mesenchymal stem cell.
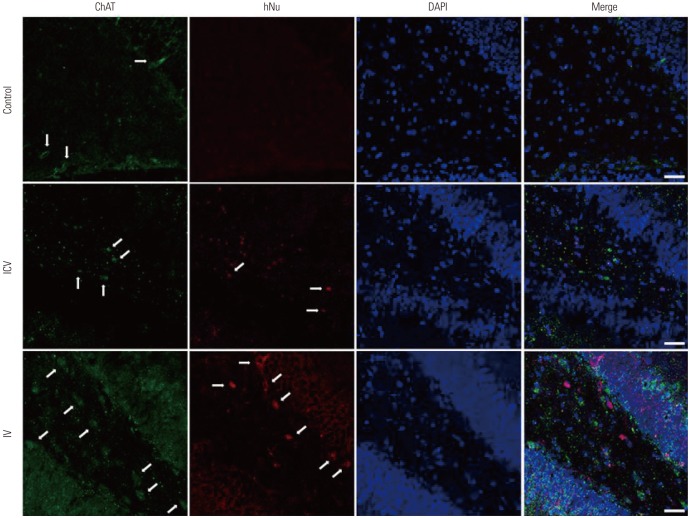
Fig. 6
Immunofluorescence analysis of the microglia at the dentate gyrus region using Iba1. (A) Samples were stained with Iba1 (green) and DAPI (blue) (×10, objective lense). The number of Iba1-positive microglial cells at the dentate gyrus significantly increased from the normal group to the lesion group. ICV and IV administrations of placenta-derived mesenchymal stem cell significantly reduced the number of microglial cells back to normal levels. Scale bar=200 µm. (B) The numbers of Iba1-positive microglial cells at the dentate gyrus region were counted for the four groups. Comparisons among groups were made using one-way analysis of variance followed by post hoc Fisher's least significant difference method. *p<0.01, †p<0.001 for comparisons. ICV, intracerebroventricular; IV, intravenous.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download