Abstract
Recurrent hyperhidrosis after thoracic sympathectomy is an uncomfortable condition, and compensatory hyperhidrosis (CH) is one of the most troublesome side effects. Here, we describe two patients with recurrent palmar hyperhidrosis (PH) and CH over the whole body simultaneously. They were treated with bilateral T4 sympathetic clipping and reconstruction of the sympathetic nerve from a T5 to T8 sympathetic nerve graft, which was transferred to the resected T3 sympathetic bed site. They reported improvements in sweating and were fully satisfied with the results. Our method can be considered as an alternative approach for patients with recurrent PH and CH.
Hyperhidrosis is characterized by excessive sweating that may be generalized or confined to the hands, axillae, face, and feet. Patients with hyperhidrosis are frequently embarrassed because of sweaty handshakes or sweat-stained clothes, and they often have poor psychological status and social interaction.
Despite the effectiveness of endoscopic thoracic sympathectomy (ETS) for palmar hyperhidrosis (PH), it is known that recurrent hyperhidrosis with re-sweating on the palms or compensatory hyperhidrosis (CH) with excessive sweating over the whole body can occur in some patients after ETS.
We performed bilateral T4 sympathetic clipping and reconstruction of the sympathetic nerve from a T5 to T8 sympathetic nerve graft in two patients with recurrent PH and severe CH after ETS. This study was reviewed and approved by the Institutional Review Board at CHA University (IRB No. 2017-11-029). Informed consent for procedure was obtained from all participants.
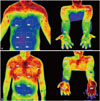
A 30-year-old man was referred for treatment of recurrent PH and CH. He had undergone T3 sympathetic clipping and T3/4 ramicotomy for PH 12 years previously. He had developed CH at 2 months after ETS and recurrent sweating on the palms at 2 years after ETS. He had failed to respond to conservative therapy with antiperspirants containing aluminum salts and iontophoresis. He had considered definitive surgical treatment. Preoperative digital infrared thermal imaging (DITI) revealed decreased temperature on the palms, anterior chest wall, and back, suggesting recurrent PH and CH.
He was intubated with a double lumen tube for general anesthesia. He was placed in the left lateral decubitus position with the right arm elevated, and the right chest wall was prepared. Four 5-mm trocars were inserted into the right thoracic cavity after the right lung was collapsed by an anesthesiologist. After exposing the sympathetic nerve, previously applied clips at the T3 sympathetic nerve were removed, and a T4 sympathetic block using titanium clips was performed just above the fourth rib. The sympathetic nerve from T5 to T8 was harvested and attached over the exposed T2 to T3 sympathetic nerve site. Nerve anastomosis was achieved with a 4-0 monofilament suture, followed by application of fibrin glue (Fig. 1). Surgery for the left side was performed in the same manner. The early postoperative course was uneventful with decreased sweating on the chest, abdomen, back, and hands. He was discharged on the fourth postoperative day. Postoperative DITI showed increased temperature on the palms, anterior chest wall, and back, suggesting improvement in hyperhidrosis. At 6 months after ETS, he continued to not experience sweating on the hands, chest, abdomen, and back, although he experienced mild sweating on the buttocks and lower extremities. He was fully satisfied with the outcome of the operation.
A 25-year-old man was referred to our hospital for the treatment of recurrent PH and CH. He had undergone T2 and T3 sympathetic ramicotomy for PH 11 years previously. He had developed CH 3 months after ETS and recurrent sweating on the palms 1 year after ETS. Because he had failed to respond to conservative therapy, surgical treatment was considered. Preoperative DITI showed decreased temperature on the palms, anterior chest wall, and abdomen, suggesting recurrent PH and CH.
Surgery was performed using 5-mm trocars as part of video-assisted thoracoscopic surgery in the same manner as the first case. Bilateral T4 sympathetic clipping and reconstruction of the sympathetic nerve from a T5 to T8 sympathetic nerve graft was performed. He was discharged on the fourth postoperative day with decreased sweating on the hands and no sweating on the chest, abdomen, and back. Postoperative DITI showed increased temperature on the palms, anterior chest wall, and abdomen, suggesting improvement in hyperhidrosis (Fig. 2). Four months after ETS, there was no recurrent hyperhidrosis.
Recurrent hyperhidrosis is the most common cause of dissatisfaction with and CH is a well-known embarrassing side effect after sympathetic surgery.12 Drott and Claes3 reported persistent sweating in 1.5% and delayed re-sweating in 2% of patients during a mean follow-up period of 31 months after ETS. The principal causes of persistent sweating or recurrent symptoms include severe pleural adhesions, existence of the nerve of Kuntz, nerve regeneration, anatomic variation of the sympathetic chain, and incomplete interruption of the sympathetic chain. The incidence of CH ranges from 3% to 100% after ETS, depending on how CH has been assessed.145 It appears that the incidence and severity of CH are high in the case of a high level of interruption and extensive destruction of the sympathetic chain.67
The chosen methods of treatment of CH depend on how localized and severe the sweating is. The current treatment options for CH include topical agents, botulinum toxin, systemic anticholinergics, clip removal, and sympathetic chain reconstruction. In severe CH, clip removal and nerve reconstruction are feasible alternative methods, with the potential for reversibility, although their efficacy is not established clearly.89 In 2010, we demonstrated that compensatory sweating resolved in nine (47%) of 19 patients after sympathetic nerve reconstruction using intercostal nerve.10
Sympathetic nerve reconstruction using either a sural or an intercostal nerve can be considered as an alternative treatment for patients with severe CH after ETS. In this study, the main reason for the use of an autologous sympathetic nerve graft was that the component of the lower sympathetic nerve is a pure autonomic nerve, but an intercostal or sural nerve has a larger somatic nerve than an autonomic nerve. Moreover, it is possible to harvest and implant a sympathetic nerve graft at the same time without position change.
We used a questionnaire and DITI to evaluate the surgical outcomes after ETS. However, the questionnaire has a limitation of non-objectivity. In addition, because CH depends on the climate and season, the timing of a questionnaire survey is important. Additionally, it is unclear whether extended lower sympathectomy or sympathetic nerve reconstruction improves the symptoms of CH, and this can be considered another limitation in this study.
In conclusion, endoscopic sympathetic block of T4 with clipping is considered as an effective treatment for recurrent PH, and reconstruction of the sympathetic nerve with a lower sympathetic nerve graft is a useful treatment for CH.
Figures and Tables
References
1. Chou SH, Kao EL, Lin CC, Chang YT, Huang MF. The importance of classification in sympathetic surgery and a proposed mechanism for compensatory hyperhidrosis: experience with 464 cases. Surg Endosc. 2006; 20:1749–1753.

2. Riet M, Smet AA, Kuiken H, Kazemier G, Bonjer HJ. Prevention of compensatory hyperhidrosis after thoracoscopic sympathectomy for hyperhidrosis. Surg Endosc. 2001; 15:1159–1162.

3. Drott C, Claes G. Hyperhidrosis treated by thoracoscopic sympathicotomy. Cardiovasc Surg. 1996; 4:788–790.

4. Bryant AS, Cerfolio RJ. Satisfaction and compensatory hyperhidrosis rates 5 years and longer after video-assisted thoracoscopic sympathotomy for hyperhidrosis. J Thorac Cardiovasc Surg. 2014; 147:1160–1163.

5. Cerfolio RJ, De Campos JR, Bryant AS, Connery CP, Miller DL, De-Camp MM, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg. 2011; 91:1642–1648.

6. Ishy A, de Campos JR, Wolosker N, Kauffman P, Tedde ML, Chiavoni CR, et al. Objective evaluation of patients with palmar hyperhidrosis submitted to two levels of sympathectomy: T3 and T4. Interact Cardiovasc Thorac Surg. 2011; 12:545–548.

7. Wait SD, Killory BD, Lekovic GP, Ponce FA, Kenny KJ, Dickman CA. Thoracoscopic sympathectomy for hyperhidrosis: analysis of 642 procedures with special attention to Horner's syndrome and compensatory hyperhidrosis. Neurosurgery. 2010; 67:652–656.
8. Lin CC, Mo LR, Lee LS, Ng SM, Hwang MH. Thoracoscopic T2-sympathetic block by clipping--a better and reversible operation for treatment of hyperhidrosis palmaris: experience with 326 cases. Eur J Surg. 1998; 164:suppl 580. 13–16.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download