Abstract
Purpose
To assess the association between frailty and osteoporotic vertebral compression fracture (OVCF) and to evaluate the relationship between numbers of OVCFs and frailty.
Materials and Methods
We enrolled 760 subjects, including 59 patients (with OVCF) and 701 controls (without OVCF). Successful matching provided 56 patient-control pairs. We analyzed principal clinical and demographic information, which included sex, age, height, weight, body mass index (BMI), variable frailty phenotypes, and Oswestry Disability Index (ODI) and EuroQol 5-dimension questionnaire (EQ-5D) scores. The association between frailty and OVCF was ascertained. In addition, the degrees of disability and quality of life attributable to frailty were determined.
Results
The prevalence of frailty was significantly higher in the OVCF group than in the control group (p<0.001). Most of the frailty phenotypes, such as exhaustion, physical inactivity, slowness, and handgrip strength, were also significantly observed in the OVCF group. Within the OVCF group, the participants with frailty had significantly higher disability and lower quality of life than those in a robust state (p<0.001 for ODI and EQ-5D). In addition, the multivariate logistic regression analysis demonstrated that the patients with low BMI [odds ratio (OR)=0.704; 95% confidence interval (CI), 0.543–0.913] and ≥3 fractures (OR=9.213; 95% CI, 1.529–55.501) within the OVCF group were associated with higher odds of frailty.
As the mean age of the general population is increasing, one of the most problematic health outcomes is the clinical condition of frailty.12 Growing research has deemed frailty a serious and increasing public health issue, which develops as a consequence of aging-related decline in many physiological systems, including physical, psychological, and social functions, collectively resulting in a clinical condition with an increased vulnerability to sudden changes in health status triggered by minor stressor events.23 This increases the risk of adverse outcomes, including falls, delirium, disability, and mortality.123
Previous studies have shown that osteoporotic fractures are associated with frailty, and frailty is further worsened after osteoporotic fractures because of deficit accumulation being greater.45 In addition, a systematic review reported that frailty and pre-frailty are significant predictors of osteoporotic fractures in older adults.6 Given that osteoporotic vertebral compression fracture (OVCF) is the most common osteoporotic fragility fracture in older adults,5 a possible association may exists between OVCF and frailty. Nonetheless, frailty in patients with vertebral fragility fractures has not been robustly studied.
We hypothesized that OVCF would be significantly associated with frailty and that a positive correlation exists between frailty and numbers of OVCFs. The purpose of this study was to assess the association between frailty and OVCF and to evaluate the relationship between numbers of OVCFs and frailty.
This propensity score-matched case-control study was performed within the framework of a prospective study designed to develop criterion-referenced health-related fitness standards for the National Fitness Award.7 This study was approved by the Institutional Review Boards of Seoul National University Bundang Hospital and Korea Institute of Sport Science, and was conducted in accordance with the approved study protocol (IRB No. B-1612-373-301). All participants provided written informed consent before study participation. For the study, 760 participants were enrolled, including 59 patients with a diagnosis of OVCF (OVCF group) and 701 healthy individuals without any osteoporotic compression fracture or back pain (control group). The patients with OVCF were recruited from a single center of a tertiary-care teaching hospital and the control subjects from either the National Fitness Award or community centers from August 2014 to February 2017. The eligibility criteria for the OVCF group were as follows: age of 65 to 85 years and an old vertebral compression fracture caused by a minor trauma at least 6 months prior. We defined osteoporotic vertebral fracture as an axial compression of the vertebral body with intact posterior constraining elements, which included wedge, biconcavity, and compression deformity as described by Eastell, el al.8 The inclusion criteria for the healthy control group were as follows: age of 65 to 85 years, absence of low back pain, and no history of OVCF. The main exclusion criteria for both groups were as follows: any neurological deficit caused by OVCF; severe joint pain impeding walking; presence of peripheral vascular diseases; any clinically significant medical comorbidity, such as sepsis, which might influence the general medical condition of the patients; and cancer. Study subjects with incomplete questionnaire findings were excluded from the study.
For each subject, the following baseline clinical and demographic variables were collected: sex, age, height, weight, body mass index (BMI), variables regarding frailty assessment, medical history, and clinical outcomes, including the Oswestry Disability Index (ODI) and the EuroQol 5-dimension questionnaire (EQ-5D).91011 Bone mineral densities at the lumbar spine and hip joints were measured using a dual-energy absorptiometry scan. In addition, radiographic images were obtained in the standing position.
Frailty was defined in accordance with the following five phenotypes as reported by Fried, et al.12 (Table 1): weight loss, exhaustion, physical inactivity, slowness, and handgrip strength.
Exhaustion was assessed using the following question from the Center for Epidemiological Studies-Depression Scale:12 A) “I felt that everything I did was an effort” and B) “I could not get going.” If answered yes, the following question was asked: “How often in the previous week did you feel this way?” Scores ranged from 0 to 3, where 0 indicated rarely or none of the time (1 day), 1 indicated some of the time (1–2 days), 2 indicated a moderate amount of the time (3–4 days), and 3 indicated most of the time. The subjects answering 2 or 3 to either of these questions were categorized as frail, as per exhaustion criterion.
For physical activity, the International Physical Activity Questionnaire-Short Form was used.13 The participants were asked regarding the amount of time they spent engaged in physical activities during the past week, and those in the lowest quintile were defined as physically inactive.
Slowness was defined as the slowest quintile of the walking performance of the subjects at 5 m. Adjustments were made for the sex and standing height of the patients.
For the handgrip strength measurement, the participants were instructed to squeeze a handgrip dynamometer (GRIP-D5101; Takei, Niigata, Japan) as hard as possible; this exercise was repeated thrice (once with each hand and then with the strongest hand), and the maximum value was recorded. Sex- and BMI-specific cutoff values for grip strength were used to identify the subjects with frailty.12 The subjects who did not fulfill any criteria for frailty were considered robust, and those who fulfilled one or two criteria were considered pre-frail. If three or more frail phenotypes existed in the subjects, then they were considered frail.
The ODI is a self-administered questionnaire that measures back-specific function on a 10-item scale with six response categories for each item. Each item is scored from 0 to 5, and the summation of scores for each item is converted into a 0??00 scale.9 The EQ-5D-5L is a five-dimensional health-state classification.11 The five dimensions are mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. An EQ-5D-5L health status is defined by selecting one level from each dimension. The EQ-5D-5L preference-based measure can be regarded as a continuous outcome scored in a scale of 0??.00, with 1.00 indicating full health and 0 representing death.11
The t-test and chi-square test were used to analyze unadjusted continuous and categorical variables. To adjust the differences in the baseline characteristics and influential factors for frailty, a propensity score-matched algorithm was used. The propensity score was estimated using a logistic regression model to balance the baseline covariates between the two groups.14 For this study, one-to-one matching was used with a nearest neighbor matching without replacements, in which each case in the control group was matched with a unique case in the OVCF group based on the nearest propensity scores. Factors associated with frailty, such as age, sex, BMI, education level, and income, were considered as confounders, and used in the logistic regression analysis.2151617 This procedure produced 56 wellmatched pairs in the OVCF and control groups. Three subjects in the OVCF could not be matched with those in the control group because of a fairly large discrepancy in the propensity scores of three subjects between both groups.
In the propensity score-adjusted groups, the baseline clinical and demographic variables were analyzed using the t test and chi-square test for categorical and continuous data analyses. The adjusted prevalence of frailty was compared between the OVCF and control groups. Given the difference in the prevalence of frailty between the two groups, the post hoc power was also calculated. The Kruskal-Wallis test with post hoc Bonferroni-corrected Mann-Whitney tests was used for the comparison of the clinical outcomes and other variables within each study group. Furthermore, the analysis of covariance was conducted to stratify age and sex.
In the OVCF group, the chi-square test was used to analyze the association between the numbers of OVCF and frailty. To adjust for the confounders, such as age, BMI, and sex, univariate and multivariate models were created. The variables that were significantly associated with frailty at p values of <0.10 in the univariate analysis were entered in the multivariate model, along with potentially important variables, including age, BMI, and sex, regardless of their statistical significance. For the multivariate model, we anticipated a potential issue of collinearity between the variables and set an a priori rule to exclude variables with correlation coefficients of ≥0.50. The alpha level of significance was set at 0.05. All statistical analyses were performed using the SPSS version 20.0 software (IBM Corp., Armonk, NY, USA), except the post hoc power analysis. Given the difference in the prevalence of frailty between the groups, a post hoc power analysis was also performed in 56 matched cases and controls, with an alpha value of 0.05 using G*power 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007).
In total, 760 subjects were recruited in the study, including 59 patients in the OVCF group and 701 individuals in the control group (Fig. 1). Table 2 demonstrates the baseline characteristics of the participants in both groups. Significant differences were found in sex distribution and income (p<0.001 and p=0.022, respectively). As expected, the clinical outcomes, including the ODI and EQ-5D findings, were significantly worse in the OVCF group than in the control group (p<0.001 for both variables). Furthermore, the subjects in the OVCF group had a significantly higher percent ratio of frail and pre-frail states than the control group (p<0.001). In the OVCF group, all patients had a vertebral fracture from T7 to L5, and the most frequent fractured level was L1 in 27 patients. Twenty-eight patients (50%) had multiple OVCFs.
The propensity score matching yielded 56 well-matched patients with OVCF and control pairs (Fig. 1). After matching, no significant difference was found between the two groups in terms of age, sex, BMI, educational level, and income. However, the OVCF group demonstrated significantly higher disability (ODI) and lower health-related quality of life (EQ-5D findings; p<0.001 for both variables) than the control group.
Table 3 shows that the OVCF group had a significantly higher prevalence of frailty than the control group (p<0.001); 24 (42.9%) and 22 participants (39.3%) in the OVCF group and 2 (3.6%) and 24 participants (42.9%) in the control group were considered frail and pre-frail, respectively (Table 3). The post hoc power analysis confirmed this difference in mean and standard deviation in the ratio of frail to pre-frail and robust participants, with an alpha value of 0.05 and a statistical power of 100.0%. Among the frailty criteria, no significant difference in weight loss was found between the two groups, whereas the other criteria were significantly different between them (Table 3).
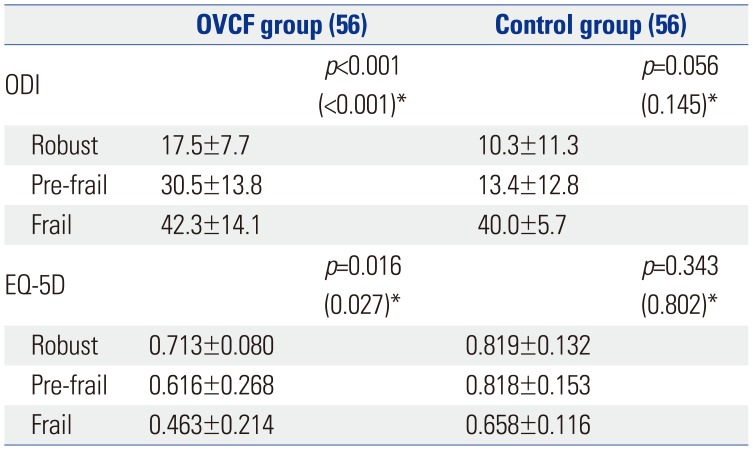
Within the OVCF group, the participants with frailty had significantly higher disability and lower quality of life than those in a robust state (p<0.001 for ODI and EQ-5D) (Table 4). The post hoc analysis with Bonferroni correction for the ODI and EQ-5D scores showed significant differences between the frail and pre-frail patients (p=0.034 and p=0.032, respectively) and between the frail and robust patients (p<0.001 and p=0.004, respectively) in the OVCF group. After adjustments for age and sex, the ODI and EQ-5D were significantly different with regard to the frailty status in the OVCF group (Table 4).
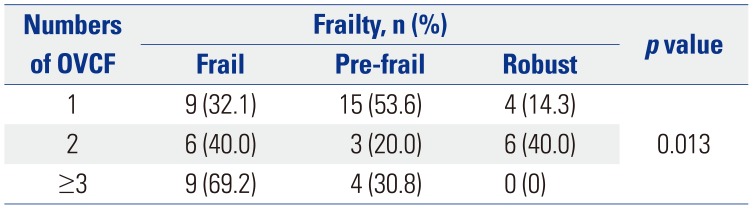
Table 5 demonstrates the association between the numbers of OVCFs and frailty. The patients who had ≥3 vertebral fractures had a significantly higher risk for frailty (p=0.013) in the OVCF group. In the univariate logistic model, consisting of the OVCF group, education level, income, living status, alcohol habitus, smoking, and any kind of chronic disease did not have significant association with frailty at a p value of <0.1. Therefore, the final multivariate logistic analysis was performed in the OVCF group so that the impact of age, sex, BMI, and number of fractures could be assessed. It demonstrated that BMI [p=0.008; odds ratio (OR), 0.704; 95% confidence interval (CI), 0.543??.913] and the numbers of OVCFs (≥3 fractures vs. <3 fractures; p=0.015; OR, 9.213; 95% CI, 1.529??5.501) were significantly associated with lower and higher odds of frailty, respectively.
The study shows that frailty is prevalent in the patients with OVCF. More than 50% of the patients in the OVCF group were classified as “frail” according to the Fried frailty criteria. In the OVCF group, the patients with frailty had higher disability and lower health-related quality of life. Furthermore, the numbers of OVCF were significantly related to frailty.
Although frailty has emerged as a significant issue in older adults, frailty in patients with vertebral fragility fractures has been underestimated, compared with that in patients with osteoporotic hip fractures. The Global Longitudinal Study of Osteoporosis in Women (GLOW) study demonstrated that frailty, according to the Fried frailty criteria, appeared to be associated with fragility fractures, disabilities, and falls.518 However, this study included only 2% of their total cohort patients with existing vertebral fractures.18 Two previous studies investigated the association between vertebral compression fracture and frailty.519 Kado, et al.19 reported that older women with incident vertebral fractures have an increased risk of mortality, which can be explained by weight loss and physical frailty. However, their study did not use any specific frailty diagnostic criteria, but included only physical frailty markers, such as weight loss, inability to rise from a chair, and difficulty standing on the feet for 2 hours.19 A recent study by Walters, et al.5 showed that frailty is prevalent in patients hospitalized owing to vertebral fragility fractures.
The within-group analysis revealed that frailty had a stronger relationship with more severe symptoms and higher disability induced by OVCF. This result can also be explained by the fact that frailty might directly aggravate the disability and health-related quality of life of patients with OVCF. The present study could not show a causal relationship between OVCF and frailty owing to its cross-sectional design. Nonetheless, we can surmise the relationship from previous studies.41819 In fact, frailty is well known to be a significant risk factor of fall and osteoporotic fracture.6182021 Kado, et al.19 reported that physical frailty may be a risk factor of vertebral fractures, but is likely a complication that occurs after vertebral fractures. A study using data from the GLOW Hamilton 3-year cohort clearly showed that the increase in frailty index was significantly greater in the participants with major osteoporotic fractures than in those without major osteoporotic fractures, indicating increasing frailty incidences and faster deficit accumulation in older women after major osteoporotic fractures.4 Therefore, based on the results of the present and previous studies, we consider that frailty causes or results from incident vertebral fractures.4181920
In addition, the increased numbers of OVCFs were significantly associated with a higher prevalence of frailty in the OVCF group. After adjustments for age, sex, and BMI, ≥3 OVCFs were associated with higher odds for frailty than a single number of fracture. This result is in line with the results of a previous study that used the GLOW database,4 in which the increase in the frailty index was significantly greater in the older women experiencing a major osteoporotic fracture than in their peer controls. Therefore, we consider that OVCF has an accumulative effect on increasing frailty incidences and worsening deficits. However, patients with frailty might have higher odds for multiple compression fractures.
This study had several limitations. First, the sample size in the OVCF group was relatively smaller than that in the control group. Although the post hoc power was 100%, such a relatively small sample size in the OVCF group might have led to a selection bias. Second, the present study could not reveal the causal relationship between OVCF and frailty, owing to the cross-sectional design of the study. However, we think that OVCF would be a cause and/or result of frailty. Future longitudinal studies may provide some values to the existing evidence on frailty and OVCF. Third, the relationship between sagittal alignment, including lumbar lordosis and kyphosis, and ODI/EQ-5D should have been analyzed. However, because radiography was obtained only at the fracture level, the global and focal alignment could not be measured.
In conclusion, the present study demonstrates a significant association between OVCF and frailty. Clinical outcomes, such as disability and health-related quality of life, were significantly associated with frailty in the OVCF group. Therefore, as hip fractures have traditionally been regarded to represent frailty,46182122 proper attention and management of both frailty and OVCF are necessary because both have reciprocal interaction, that is worsening frailty deficits by fracture and accelerated risk of OVCF by frailty.
ACKNOWLEDGEMENTS
This study was supported by research grants from the Korea Sports Promotion Foundation, Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2016R1A2B3012850), and research grants from Hanlim Pharm.
References
1. Keevil VL, Romero-Ortuno R. Ageing well: a review of sarcopenia and frailty. Proc Nutr Soc. 2015; 74:337–347. PMID: 26004622.

2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013; 381:752–762. PMID: 23395245.

3. Cooper Z, Rogers SO Jr, Ngo L, Guess J, Schmitt E, Jones RN, et al. Comparison of frailty measures as predictors of outcomes after orthopedic surgery. J Am Geriatr Soc. 2016; 64:2464–2471. PMID: 27801939.

4. Li G, Papaioannou A, Thabane L, Cheng J, Adachi JD. Frailty change and major osteoporotic fracture in the elderly: data from the global longitudinal study of osteoporosis in women 3-year hamilton cohort. J Bone Miner Res. 2016; 31:718–724. PMID: 26547825.

5. Walters S, Chan S, Goh L, Ong T, Sahota O. The prevalence of frailty in patients admitted to hospital with vertebral fragility fractures. Curr Rheumatol Rev. 2016; 12:244–247. PMID: 27323881.

6. Kojima G. Frailty as a predictor of fractures among community-dwelling older people: a systematic review and meta-analysis. Bone. 2016; 90:116–122. PMID: 27321894.

7. Park S, Kim HJ, Ko BG, Chung JW, Kim SH, Park SH, et al. The prevalence and impact of sarcopenia on degenerative lumbar spinal stenosis. Bone Joint J. 2016; 98-B:1093–1098. PMID: 27482023.

8. Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ 3rd. Classification of vertebral fractures. J Bone Miner Res. 1991; 6:207–215. PMID: 2035348.

9. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000; 25:2940–2952. PMID: 11074683.

10. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997; 35:1095–1108. PMID: 9366889.

11. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001; 33:337–343. PMID: 11491192.
12. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–M156. PMID: 11253156.
13. Chun MY. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med. 2012; 33:144–151. PMID: 22787536.

14. Kim RB, Garcia RM, Smith ZA, Dahdaleh NS. Impact of resident participation on outcomes after single-level anterior cervical diskectomy and fusion: an analysis of 3265 patients from the american college of surgeons national surgical quality improvement program database. Spine (Phila Pa 1976). 2016; 41:E289–E296. PMID: 26555830.
15. Theou O, Brothers TD, Rockwood MR, Haardt D, Mitnitski A, Rockwood K. Exploring the relationship between national economic indicators and relative fitness and frailty in middle-aged and older Europeans. Age Ageing. 2013; 42:614–619. PMID: 23443511.

16. Chang YW, Chen WL, Lin FG, Fang WH, Yen MY, Hsieh CC, et al. Frailty and its impact on health-related quality of life: a cross-sectional study on elder community-dwelling preventive health service users. PLoS One. 2012; 7:e38079. PMID: 22662268.

17. Hoogendijk EO, van Hout HP, Heymans MW, van der Horst HE, Frijters DH, Broese van Groenou MI, et al. Explaining the association between educational level and frailty in older adults: results from a 13-year longitudinal study in the Netherlands. Ann Epidemiol. 2014; 24:538–544. PMID: 24935466.

18. Tom SE, Adachi JD, Anderson FA Jr, Boonen S, Chapurlat RD, Compston JE, et al. ; GLOW Investigators. Frailty and fracture, disability, and falls: a multiple country study from the global longitudinal study of osteoporosis in women. J Am Geriatr Soc. 2013; 61:327–334. PMID: 23351064.
19. Kado DM, Duong T, Stone KL, Ensrud KE, Nevitt MC, Greendale GA, et al. Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int. 2003; 14:589–594. PMID: 12827222.

20. Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005; 53:1321–1330. PMID: 16078957.
21. Liu LK, Lee WJ, Chen LY, Hwang AC, Lin MH, Peng LN, et al. Association between frailty, osteoporosis, falls and hip fractures among community-dwelling people aged 50 years and older in taiwan: results from I-Lan Longitudinal Aging Study. PLoS One. 2015; 10:e0136968. PMID: 26348034.

22. Tucker A, Donnelly KJ, McDonald S, Craig J, Foster AP, Acton JD. The changing face of fractures of the hip in Northern Ireland: a 15-year review. Bone Joint J. 2017; 99-B:1223–1231. PMID: 28860404.
Fig. 1
Enrollment, group assignment, and propensity score matching. OVCF, osteoporotic vertebral compression fracture.
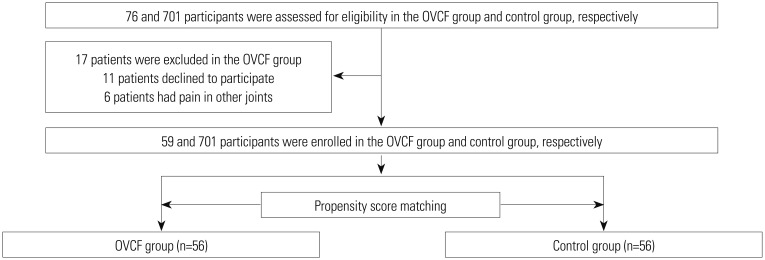
Table 1
Frailty-Defining Criteria Used in this Study
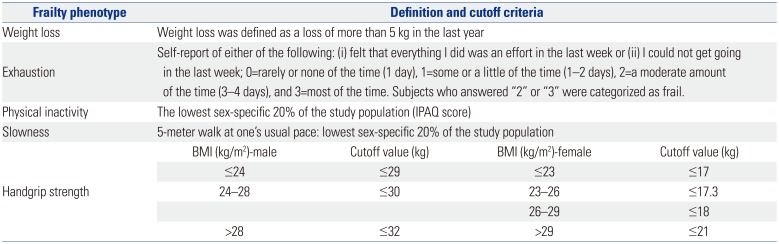
Table 2
Descriptive Statistics of the Subjects in the Unadjusted Population

Table 3
Descriptive Statistics of the Subjects in the Propensity Score-Matched Case-Control Group
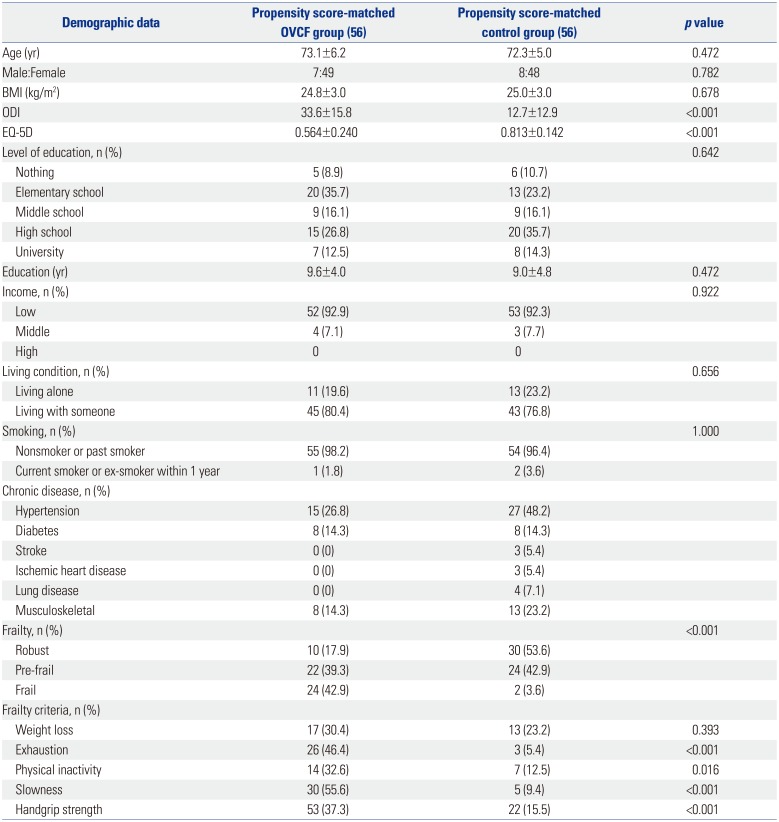
Table 4
Clinical Outcome Variables according to Frailty Status in Each Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download