Abstract
Purpose
Gastric cancer shows a male predominance that might be explained by protective effects from estrogens in females. Two Lauren classification histological subtypes, intestinal and diffuse, have distinct carcinogeneses. The purpose of this study was to estimate the effects of sex hormone on female gastric cancer according to Lauren classification.
Materials and Methods
We reviewed medical records for and administered questionnaires, surveying reproductive and hormonal factors, to 758 patients who underwent gastrectomy for gastric cancer at Samsung Medical Center from May 2012 to November 2014. Clinicopathological characteristics were compared between females and males. The incidence of intestinal-type gastric cancer was compared between females subgroups, consist of premenopausal women and three groups of postmenopausal women (five-year intervals after menopause), and males. The association between reproductive factors and intestinal-type gastric cancer was analyzed by multivariate models for the female group.
Results
In total, 227 females (29.9%) and 531 males (70.9%) were included in the analysis. Undifferentiated adenocarcinoma and diffuse-type histology were more frequent in female patients than male patients. While 221 (41.6%) male patients had intestinal-type gastric cancer, no premenopausal female patient had this type of gastric cancer. The incidence of intestinal-type gastric cancer increased with time after menopause, and was similar to males after 10 years from menopause. Parity was associated with an increased risk of intestinal-type gastric cancer in menopausal women.
Gastric cancer is the fifth most common cancer and the third leading cause of cancer related death worldwide.1 Gastric cancer shows male dominance at a male-to-female ratio of about 2:1.2 This male predominance cannot be clearly explained by known risk factors.
Several studies have reported that the female sex hormone estrogen may be associated with gastric carcinogenesis.34 17β-estradiol (E2) down-regulates E-cadherin through Estrogen Receptor (ER)-α,5 which may initiate diffuse-type gastric cancer. However, the significance or mechanism of estrogen in gastric cancer has not been established.
Gastric cancer can be classified into two histologic subtypes, intestinal and diffuse, which are distinct in their microscopic and gross appearance, epidemiology, pathogenesis, and prognosis.6 Diffuse-type gastric cancer occurs more in female, younger patients and in patients who are diagnosed at an advanced stage, and the prognosis is worse, compared to intestinal-type gastric cancer.7 Diffuse-type gastric cancer shows different patterns, such as TP53 mutation and ERBB2 or ERBB3 overexpression in gastric cancer signaling pathway, from intestinal type. Gastric cancer also shows distinct features according to age. A study revealed that early onset gastric cancers are different from cases diagnosed in older adults in terms of clinicopathological factors and molecular profiles in each type of Lauren classification.8 The different patterns of gastric cancers according to gender and age imply the possible role of sex hormones in carcinogenesis, and it should be considered in diffuse and intestinal type. The purpose of this study was to estimate the effects of sex hormone on female gastric cancer according to Lauren classification.
A total of 3791 patients had undergone gastrectomy for gastric cancer at Samsung Medical Center from May 2012 to November 2014 (Fig. 1). We screened 1506 patients, and 722 patients had refused to participate in the survey. Twenty-six female patients who did not complete the reproductive factor-related questionnaires were excluded. We reviewed medical records for 758 patients who completed a questionnaire including menstrual and reproductive history. Patients who met the following criteria were included in analysis: histologically confirmed adenocarcinoma of the stomach; radical resection of the tumor; and females who provided obstetric and menstruation history. All information was obtained with the appropriate Institutional Review Board waivers, and data were collected without revealing any personal information (IRB File No. 2012-05-096).
All patient characteristics were obtained from retrospective review of a prospectively maintained database. Sociodemographic characteristics included age, sex, body mass index, and smoking and drinking history. Clinicopathological characteristics included tumor size, tumor location, depth of invasion, lymph node metastasis, TNM stage at diagnosis, lymphatic invasion, vascular invasion, perineural invasion, differentiation, and Lauren classification. Stage at diagnosis was determined in accordance with the seventh edition of the Union International Centre Cancer/American Joint Committee on Cancer classification system.9 Reproductive factors included age at menarche, menopausal status, and if relevant, age at menopause, number of full-term pregnancies and woman's age at birth of first child, breastfeeding history, postmenopausal hormonal therapy, oral contraceptives, hysterectomy, and oophorectomy.
The primary endpoint of the study was the association between female reproductive factors and intestinal-type gastric cancer by Lauren classification.6 Lauren classification divides gastric cancer into intestinal or diffuse types according to pathologic findings. Mixed type showing both intestinal and diffuse types was regarded as diffuse type.
Chi-squared test and Fisher's exact test were used to evaluate the associations among categorical variables, and Student's t-test was used for continuous variables including age and tumor size. Multivariate analysis was carried out using a logistic regression method. All statistical analyses were performed using SPSS version 23.0 for Windows (IBM Corp., Armonk, NY, USA), and p-values less than 0.05 were considered significant.
Seven hundred and fifty-eight patients with gastrectomy for gastric cancer were included in the final analysis: 227 were females (29.9%) and 531 were males (70.9%). Baseline characteristics according to sex are shown in Table 1. Most female patients had no drinking history (75.8%) or smoking history (95.1%), whereas there were 435 male patients (81.9%) who had drinking history and 432 male smokers (81.4%). For past and current smokers, tobacco exposure was higher in male than female patients. 446 patients (58.8%; 137 female and 309 male) had early gastric cancer, and 312 patients (41.2%; 90 female and 222 male) had advanced gastric cancer. Male patients showed more frequent lower located gastric cancer and vascular invasion, compared to female patients. Undifferentiated adenocarcinoma and diffuse-type histology were more frequent in female patients, compared to male patients. Of male patients, 41.6% (221/531) had gastric cancer classified as intestinal type and 58.4% (310/531) had gastric cancer classified as diffuse type according to Lauren classification. Of female patients, 21.6% (49/227) had gastric cancer specified as intestinal type and 78.4% (178/227) as diffuse type. There were 115 patients with mixed-type gastric cancer (81 in male and 34 in female).
The proportions of gastric cancer subtypes in female subgroups classified by five-year intervals after menopause are shown in Fig. 1. 66 premenopausal women and 98 women with menopause over 10 years were included in this analysis. No premenopausal women had intestinal-type gastric cancer; in contrast, 37.8% (37/98) women with menopause over 10 years had intestinal-type gastric cancer (Fig. 2). Intestinal-type gastric cancer increased in postmenopausal female gastric cancer patients with time after menopause (Fig. 3).
Of male patients, 221 (41.6%) had intestinal-type gastric cancer, while no premenopausal female patients did (Fig. 4). The incidence of intestinal-type histology was higher in postmenopausal female patients, however. The proportions of diffuse-type gastric cancer in male and female groups are presented in Fig. 5. The proportions of intestinal-type gastric cancer showed an upward trend with time after menopause in female patients with gastric cancer. Intestinal-type gastric cancer was significantly less frequent in premenopausal female (p<0.001), postmenopausal women (p<0.001), and women who had finished menstruation more than five years previously (p=0.013), compared to male gastric cancer patients. No significant difference was detected between men and women who went through menopause more than 10 years prior (p=0.310).
We examined the association between reproductive factors and intestinal-type gastric cancer using multivariate models adjusted for categorical variables for all females and two subgroups: parous women and postmenopausal women. For all female patients with gastric cancer, only smoking history was associated with intestinal-type gastric cancer (Table 2). Alcohol history (p=0.009), menopause (p<0.001), and parity (p<0.001) were significantly associated with gastric cancer subtype in univariate analysis, although they had no significant association in multivariate analysis.
For parous women, smoking history also had a relationship with intestinal-type gastric cancer in multivariate analysis (Table 3). Menopause and reproductive factors, including parity and breast feeding, were related to intestinal-type gastric cancer in univariate analysis, although they did not have significant effects in multivariate analysis.
For postmenopausal women, an increased risk of intestinal type gastric cancer was observed for those with smoking history or parity on uni- and multivariate analysis. No significant associations were observed for other menstrual and reproductive factors (Table 4).
Estrogens regulate the growth, differentiation, and function of several tissues, and their effects are mediated by ER-α and ER-β. ER-α is expressed in female sex organs, such as the breast, uterus, and ovaries, while ER-β is more widely distributed in the body than ER-α.10 The oncologic significance of estrogens and ERs in cancers arising in breast, ovary, and uterine endometrium has been well described.111213 Estrogen is associated with an increased risk for breast14 and endometrial15 cancer and a decreased colon cancer risk.16
Estrogen and estrogen receptor have been suspected as playing a role in gastric cancer because of a global pattern of male predominance that is unique for intestinal-type gastric cancer. Some investigators have regarded estrogen as preventive against gastric cancer, as men who receive estrogen for prostate cancer show a reduced risk of gastric cancer.17 Studies show that tamoxifen exposure is a risk factor for gastric cancer, further supporting the hypothesis.18 Furukawa, et al.19 had reported that the carcinogenic N-methyl-N0-nitro-N-nitrosoguanidine added to drinking water induced gastric cancer in male rats but not in female rats, and castrated or estrogen-treated male rats had a lower incidence of gastric cancer than untreated male rats in in vitro experiments. In addition, some studies propose that estrogen has a protective role, since menopause at older age is associated with decreased gastric cancer.3
It has been reported that diffuse-type gastric cancer is predominant in younger gastric cancer patients. Nevertheless, it is worthy to note that we could not find any intestinal-type gastric cancer in premenopausal females in this study. Although the sample size was small, this finding strongly suggests that intestinal-type gastric cancer is infrequent in women until menopause. The incidence of intestinal-type gastric cancer increased with time after menopause and became similar to that for men at 10 years after menopause. In addition, parity, which results in lower lifelong estrogen exposure than null-parity, was associated with an increased risk of intestinal-type gastric cancer for menopausal women. This finding suggests that estrogen might protect women against intestinal-type gastric cancer.
Several studies demonstrated sex disparities in different age groups and histological differentiation groups of patients with gastric cancer.2021 Sipponen, et al.20 suggested that a delay in the appearance and onset of intestinal-type gastric cancer in females relative to males results in male predominance, raising the hypothesis that female sex hormones potently prevent or inhibit carcinogenesis of intestinal-type gastric cancer. Chandanos, et al.,22 in a population-based cohort study, reported that endogenous estrogen exposure is associated with a lower frequency of intestinal-type cancers. Patients were categorized into three groups: exposed women (aged younger than 50), unexposed men (aged younger than 50), and unexposed women (aged over 70) according to age. In our study, we administered a questionnaire designed to survey detailed information on menstrual status, including time from menopause, so sex hormone effects could be analyzed by time after menopause. To the best of our knowledge, this is the first study to address the study topic with a prospective survey of reproductive factors for gastric cancer patients.
We found that smoking was associated intestinal-type gastric cancer in females, including both parous women and menopausal women. Although several studies suggest an effect of smoking on gastric cancer, published evidence is insufficient to draw conclusions. In addition, the smoking effect might not be acceptable in this study, because of the small number of female smokers (8/221 patients). In our study, undifferentiated type cancers were more frequent in the female population, probably because most of diffuse-type cancers would be categorized into undifferentiated type adenocarcinoma in WHO classification. Vascular invasion was more frequent in the male population; however, it was significantly associated with Lauren intestinal-type cancer but not with males in multivariate analysis (data not shown).
Helicobacter pylori (H. pylori) infection is a well-established risk factor and shows male-dominant features. Despite a high prevalence in Korea, we were unable to adjust for H. pylori infection because of the lack of H. pylori array for gastric cancer patients in this institution. However, Freedman, et al.3 reported pylori cytotoxin-associated gene A (CagA) seropositivity is not a confounding factor in a large prospective study of reproductive factors and gastric cancer risk. Recent meta-analysis has supported the little contribution of sex differences in the prevalence of H. pylori infection to the male predominance of gastric cancer.23 Further research is needed to clarify the effect of H. pylori according to gastric cancer subtype.
This study had the limitation of being a clinicoepidemiological study without investigating the molecular mechanism of female sex hormones. Further studies are also needed to elucidate the biological role of estrogen or estrogen receptor in the development of female gastric cancers. However, estrogen receptor has not been found to be well expressed in gastric cancer tissue, and no validated ER-α immunohistochemistry protocol or interpretation guidelines exist for gastric cancer.24
Future study should focus on subtypes and the genomic pathways of estrogen and its receptors on gastric cancer. Also, long-term outcomes including recurrence or survival could provide more information for effects of reproductive factors on gastric cancer. In addition, mixed type gastric cancer should be classified separately in a large volume database.
In conclusion, female sex hormones might be protective against intestinal-type gastric cancer. Further studies including large cohort surveys and molecular experiments will be necessary to clarify the significance and mechanism of estrogen in gastric cancer.
Figures and Tables
 | Fig. 2Number of intestinal and diffuse subtype gastric cancers in male and female subgroups classified according to cumulative 5-year intervals after menopause. *p-value was calculated compared to male groups. |
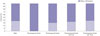 | Fig. 3Proportion of intestinal and diffuse subtype gastric cancers in male and female subgroups classified according to cumulative 5-year intervals after menopause. |
 | Fig. 4Number of intestinal and diffuse subtype gastric cancers in male and female subgroups classified according to 5-year intervals after menopause. |
 | Fig. 5Proportion of intestinal and diffuse subtype gastric cancers in male and female subgroups classified according to 5-year intervals after menopause. |
Table 1
Clinicopathological Features of Patients with Gastric Cancer
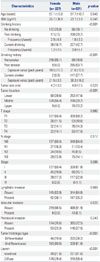
Table 2
Multivariate Analysis for Intestinal-Type Gastric Cancer Risk in All Female Gastric Cancer Patients

Table 3
Multivariate Analysis for Intestinal-Type Gastric Cancer Risk in Parous Women with Gastric Cancer
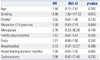
Table 4
Multivariate Analysis for Intestinal-Type Gastric Cancer Risk in Postmenopausal Women with Gastric Cancer
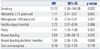
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.

2. Forman D BF, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, et al. Cancer incidence in five continents, CI5plus. IARC CancerBase No. 9. Lyon: International Agency for Research on Cancer;2014.
3. Freedman ND, Chow WH, Gao YT, Shu XO, Ji BT, Yang G, et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007; 56:1671–1677.

4. Chandanos E, Lindblad M, Jia C, Rubio CA, Ye W, Lagergren J. Tamoxifen exposure and risk of oesophageal and gastric adenocarcinoma: a population-based cohort study of breast cancer patients in Sweden. Br J Cancer. 2006; 95:118–122.

5. Helguero LA, Lindberg K, Gardmo C, Schwend T, Gustafsson JA, Haldosén LA. Different roles of estrogen receptors alpha and beta in the regulation of E-cadherin protein levels in a mouse mammary epithelial cell line. Cancer Res. 2008; 68:8695–8704.

6. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965; 64:31–49.
7. Ribeiro MM, Sarmento JA, Sobrinho Simöes MA, Bastos J. Prognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinoma. Cancer. 1981; 47:780–784.

8. Gurzu S, Kadar Z, Sugimura H, Bara T, Bara T Jr, Halmaciu I, et al. Gastric cancer in young vs old Romanian patients: immunoprofile with emphasis on maspin and mena protein reactivity. APMIS. 2015; 123:223–233.

9. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.
10. Yang H, Sukocheva OA, Hussey DJ, Watson DI. Estrogen, male dominance and esophageal adenocarcinoma: is there a link? World J Gastroenterol. 2012; 18:393–400.

11. Beral V. Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003; 362:419–427.

12. Beral V, Bull D, Reeves G. Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005; 365:1543–1551.

13. Lacey JV Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002; 288:334–341.

14. Poulter NR, Meirik O, Chang CL, Farley TMM, Kelaghan J, Marmot MG, et al. Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997; 349:1202–1209.
15. Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995; 85:304–313.

16. Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. 1999; 106:574–582.

17. Lindblad M, Ye W, Rubio C, Lagergren J. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2004; 13:2203–2207.
18. Curtis RE, Boice JD Jr, Shriner DA, Hankey BF, Fraumeni JF Jr. Second cancers after adjuvant tamoxifen therapy for breast cancer. J Natl Cancer Inst. 1996; 88:832–834.

19. Furukawa H, Iwanaga T, Koyama H, Taniguchi H. Effect of sex hormones on the experimental induction of cancer in rat stomach-a preliminary study. Digestion. 1982; 23:151–155.

20. Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002; 5:213–219.

21. Qiu MZ, Wang ZQ, Zhang DS, Luo HY, Zhou ZW, Wang FH, et al. Clinicopathological characteristics and prognostic analysis of gastric cancer in the young adult in China. Tumour Biol. 2011; 32:509–514.

22. Chandanos E, Rubio CA, Lindblad M, Jia C, Tsolakis AV, Warner M, et al. Endogenous estrogen exposure in relation to distribution of histological type and estrogen receptors in gastric adenocarcinoma. Gastric Cancer. 2008; 11:168–174.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download