Abstract
Purpose
To evaluate parameters for determining repeat prostate biopsy in patients with 5α-reductase inhibitor (5ARI) treatment after initial negative biopsy.
Materials and Methods
From January 2007 to December 2015, patients who underwent a repeat prostate biopsy after an initial negative biopsy were enrolled from multiple institutions. Serial prostate-specific antigen (PSA) levels after the initial biopsy were analyzed for PSA kinetics. Clinicopathologic variables were evaluated according to the use of 5ARIs after the initial negative biopsy.
Results
Of 419 patients with initial negative biopsies (median age=67.0 years, median PSA=6.31 ng/mL), 101 patients (24.1%) were diagnosed with prostate cancer at the repeat biopsy. An increase in PSA level at 18 months, compared to that at 6 months, was a predictor of a positive repeat biopsy. However, the use of 5ARIs was not identified as a predictor. Of 126 patients receiving 5ARI treatment after the initial biopsy, 30 (23.8%) were diagnosed with prostate cancer at the repeat biopsy. Increase in PSA level at more than two time points after 6 months of 5ARI treatment (odds ratio=4.84, p=0.005) was associated with cancer detection at the repeat biopsy. There were no significant 5ARI group-related differences in the detection rates of prostate and high-grade cancers (Gleason score ≥7).
Although prostate-specific antigen (PSA) levels are generally used in practice and are a serum marker that revolutionized the early detection and management of prostate cancer, its use is fraught with challenges due to a relative lack of cancer specificity.12 PSA levels can be elevated for numerous reasons, including a large prostate volume, severe voiding symptoms, urinary retention, infection, recent instrumentation, ejaculation, and trauma. On the other hand, 20−30% of cancers may be missed at the initial prostate biopsy.3 Furthermore, the interpretation of regular follow-up PSA levels in patients with an initial negative biopsy requires a different approach from that used for the general population, potentially resulting in an intervening repeat biopsy in individuals persistently suspected for prostate cancer.
In addition, treatment with 5α-reductase inhibitors (5ARIs), such as dutasteride and finasteride, may be considered for reducing voiding symptoms in patients with an initial negative biopsy. However, the interpretation of PSA level in terms of discriminating prostate cancer is more complex in patients treated with 5ARIs than in those who have not been treated with 5ARIs.4 For example, the serum PSA level decreases by about 50% within 6−12 months of 5ARI treatment.5 Thus, information on the use and duration of 5ARI treatment should be additionally considered in deciding on a repeat prostate biopsy. Unfortunately, there are no guidelines specifying the parameters to be considered for repeat prostate biopsy in men taking 5ARIs. As an attempt to overcome these limitations, the use of several parameters, including PSA density, percentage of free PSA, PSA velocity (PSAV), magnitude of PSA change, and the number of PSA increases after 6 months of 5ARI treatment, has been suggested in prior studies.46789
Clinicians face a dilemma when deciding to use 5ARIs or not in patients with an initial negative biopsy, as controversy exist. In cases of missed prostate cancer at the initial prostate biopsy, the use of 5ARIs might be feasible, considering the effects of chemoprevention on prostate cancer. However, patients using 5ARIs have been reported to have high-grade features (Gleason score ≥7) at diagnosis of prostate cancer, which is problematic for urologists.10111213 The aim of the present multi-insti-tutional study was to identify the parameters that recommend repeat biopsy in persistent suspected prostate cancer patients undergoing 5ARI treatment.
Approval was obtained from the Institutional Review Boards at each institution (IRB No. 2017-0524-001) for the collection of data on all patients who underwent a repeat prostate biopsy after an initial negative biopsy. From January 2007 to December 2015, 431 patients (Severance Hospital, n=144; Gangnam Severance Hospital, n=182; and National Health Insurance Service Ilsan Hospital, n=105) were enrolled. The following clinicopathological data were collected: age, body mass index, prostate volume, PSA level during follow-up after the initial negative prostate biopsy, and pathologic results after repeat prostate biopsy. Twelve patients with a history of 5ARI withdrawal, transurethral resection of the prostate, a pathological diagnosis of prostatic intraepithelial neoplasia or atypical small acinar proliferation, or those who underwent another protocol, such as an MRI-based targeted biopsy, were excluded. The remaining 419 patients were divided into two groups based on the use of 5ARIs within 3 months after the initial negative biopsy.
Follow-up, including physical examination and PSA testing, was performed every 3−6 months. A transrectal ultrasound was performed every 1−2 years or at the time of the repeat biopsy. PSA levels for 2 years after the initial negative biopsy were analyzed for PSA kinetics. PSAV (expressed as ng/mL/year) was calculated as the real-time PSA level minus the level 6 months prior, divided by the elapsed time between the two measurements. The indication for the initial prostate biopsy was an elevated PSA level ≥4 ng/mL, consecutive PSA elevations, or an abnormal digital rectal examination. The indication for repeat biopsy depended on the clinicians' decision-making with consideration of the PSA kinetics. The number of PSA increases after 6 months of 5ARI treatment was defined as the number of times the PSA level increased from the nadir, which was the minimum PSA level attained after 6 months of treatment.
We compared the clinicopathologic variables in prostate cancer patients diagnosed at repeat biopsy between the groups. Categorical variables are reported as the number of occurrences and frequencies. Continuous variables are expressed as a median (interquartile range). Student's t-test and the Pearson chi-square test were used to evaluate differences in continuous and categorical variables, respectively. Simple and multiple logistic regressions were used, with a forward stepwise procedure. Statistical analyses were performed using SPSS, version 23.0 (IBM Corp., Armonk, NY, USA). All p-values <0.05 were considered indicative statistical significance.
The baseline characteristics of the 419 patients with an initial negative biopsy (median age=67.0 years, median PSA level= 6.31 ng/mL) are presented in Table 1. A total of 101 patients (24.1%) were diagnosed with prostate cancer at the repeat biopsy (median duration between the initial and repeat prostate biopsies=23.0 months), and 126 patients (30.1%) had received 5ARI treatment. The median PSA level at the repeat prostate biopsy was 6.12 ng/mL. Compared to those with a negative repeat biopsy, patients diagnosed with prostate cancer at the repeat biopsy were older, had lower prostate volume at the initial and repeat prostate biopsies, and had a higher proportion of patients who showed an increase in PSA levels during follow-up (compared to that at 6 months prior) (p=0.007, p<0.001, p<0.001, p=0.047, respectively). However, there were no significant differences between those with and without cancer diagnosed at the repeat biopsy in terms of receiving 5ARI treatment and PSA levels at initial and repeat biopsies. In addition, PSAVs were not statistically different at any period.
In a multivariate analysis, age [odds ratio (OR)=1.07, 95% confidence interval (CI)=1.007−1.143, p=0.029], initial prostate volume (OR=0.96, 95% CI=0.929−0.983, p=0.002), and an increase in PSA at 18 months, compared to that at 6 months (OR=3.30, 95% CI=1.358−8.025, p=0.008), were found to be predictive factors of a positive repeat biopsy. However, the use of 5ARIs was not a significant predictor of prostate cancer detection (Table 2).
Table 3 summarizes the clinical variables according to the use of 5ARIs. Patients taking 5ARIs were older, had lower PSA levels, and had larger prostate volumes at the initial biopsy, compared to that in the non-5ARI group (p=0.003, p<0.001, p<0.001, p<0.001, respectively). However, there were no significant differences in prostate cancer diagnosis rate (23.8% vs. 24.5%, p=0.747) and the proportion of patients with high-grade cancer (Gleason score ≥7) (13.5% vs. 9.9%, p=0.303).
Table 4 shows the serum PSA levels and number of patients who underwent repeat prostate biopsy in 6-month intervals, according to the use of 5ARIs. In the non-5ARI group, the median PSA level during follow-up gradually decreased. Although the median PSA level in the 5ARI group also decreased, the value at 24 months was higher than that at the previous visit. At 24 months, 57.5% of the patients in the non-5ARI group and 68.3% of the patients in the 5ARI group had undergone a repeat biopsy.
In multivariate sub-analysis of the non-5ARI group, age (OR= 1.06, 95% CI=1.020−1.110, p=0.003) and PSA level at repeat biopsy (OR=0.96, 95% CI=0.946−0.982, p<0.001) were associated with the diagnosis of prostate cancer. In addition, a multivariate analysis showed that age (OR=1.12, 95% CI=1.008−1.249, p=0.035) and increase in the PSA level at more than two time points after 6 months of 5ARI treatment (OR=4.84, 95% CI= 2.150−6.205, p=0.005) were significant predictors of prostate cancer detection in the 5ARI group. However, PSAV was a not significant predictor at any period in either group (Table 5).
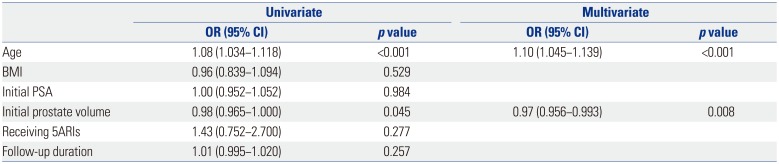
Finally, age (OR=1.10, 95% CI=1.045−1.139, p<0.001) and initial prostate volume (OR=0.97, 95% CI=0.956−0.993, p=0.008) were significant predictors of a diagnosis of high-grade prostate cancer, while the use of 5ARIs was not (Table 6).
Several studies have reported that prostate cancer detection, chemoprevention, and high-grade prostate cancer are associated with the use of 5ARIs.8121314 These results are based on a randomized controlled trial in which 5ARI was used for the purpose of relieving voiding symptoms. However, the results were controversial, as differences were present in the analysis in the same group for the risk of high-grade prostate cancer, according to the researchers.1015 Furthermore, participants in this randomized controlled trial differed from those who receive 5ARIs after an initial negative prostate biopsy in clinical practice. Without data from the actual clinical field, clinicians might be reluctant to prescribe 5ARIs to patients with large prostate volumes, which may limit the relief of voiding symptoms achieved via a synergistic effect with an α-blocker. Therefore, the present study collected data reflecting clinical practice from multiple institutions. Although the evaluation of the effects of the use of 5ARIs on prostate cancer detection and chemoprevention may not be meaningful in the present study, we can suggest an algorithm for the indication of repeat prostate biopsy to clinicians who are hesitant to prescribe 5ARIs in patients with worsening voiding symptoms. Fig. 1 presents the algorithm to recommend repeat biopsy according to PSA kinetics in patients with 5ARI treatment, based on the results of the present study.
The PSAV and an increase in the PSA level have been reported as significant predictors of a positive repeat biopsy.16171819202122 However, the definitions of these parameters were not consistent among the previous studies in terms of the regular interval for the monitoring of the PSA level. In the present study, the PSAVs calculated at 6-month intervals between the initial and repeat prostate biopsies were not predictors of prostate cancer among all patients and in the sub-groups. Instead, an increase in the PSA level at 18 months, compared to that at 6 months, was a significant predictor of positive repeat biopsy among all patients.
The influence of 5ARIs on PSA secretion by benign and malignant tissue can lead to a reduction in serum PSA levels, raising concerns about the potential of 5ARIs to interfere with detection of prostate cancer.23 5ARIs have been shown to reduce serum PSA levels by about 50% during a 6-month course.5 Although PSA level at 6 months of 5ARI treatment does not always represent the nadir, a change in PSA level at 6 months from the initial value provides an insight into the usefulness of the PSA level in prostate cancer diagnosis.1424 In the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) study and the Prostate Cancer Prevention Trial (PCPT), the percentage change at 6 months from the baseline PSA level distinguished men with prostate cancer from those without cancer during a 4-year treatment period (42% vs. 57% and 37.2% vs. 59.5%, respectively). However, the PSA level at 6 months did not reach the nadir in all patients receiving 5ARI treatment. Brawer, et al.25 reported that only 35% of the men undergoing 5ARI treatment had the expected 40−60% reduction in the PSA level. In the present study, a significant difference in the percentage change at 6 months of 5ARI treatment from the initial PSA level was found in men with and without prostate cancer (29.1% vs. 34.2%, p=0.026, data not shown), but the value among all patients was 32.4%. Thus, NCCN guidelines advise that the commonly employed method of doubling the measured PSA value to obtain an adjusted value may result in unreliable cancer detection. Kravchick, et al.26 suggested that a PSA level decline of <40% after 1 year of treatment should be considered as an indicator for repeat biopsy in patients receiving dutasteride. However, a multivariate analysis in the present study found that a PSA level decrease less than 35.0% at 6 month of 5ARI treatment was not a significant predictor for the detection of prostate cancer (p=0.700). In the present cohort, PSA level at 6 months of 5ARI treatment did not reach the nadir and the change was lower than that in previous studies. This may be related to variations in testosterone metabolism by ethnicity: Asian men have lower total serum testosterone and hormone-binding levels, reduced 5α-reductase levels, and lower testosterone production, compared to that in Western men.27
Several previous studies have suggested that an increase in serum PSA from the nadir in men receiving 5ARIs treatment should be considered as suspicious for prostate cancer. Andriole, et al.24 reported that the optimal combination of sensitivity and specificity was achieved with a PSA increase of 0.8 ng/mL as the threshold for prostate biopsy in patients using dutasteride. Marks, et al.28 reported that a PSA increase of 0.3 ng/mL from the nadir is a trigger for biopsy in men using dutasteride. The use of any increase in PSA with dutasteride as a reason for repeat biopsy resulted in a lower specificity than that achieved when attempting to confirm the nadir value and any subsequent increase in men with a previous negative biopsy.20 In the present study, an increase in the PSA level at more than two time points after 6 months of 5ARI treatment was associated with the detection of prostate cancer at repeat biopsy (OR= 4.84, p=0.005). However, a multivariate analysis showed that increase in PSA level at 24 months, compared with that at 6 months, was not a significant predictor for the diagnosis of prostate cancer in patients undergoing 5ARI treatment (p=0.381).
The effect of 5ARIs on preventing or delaying the appearance of prostate cancer remains controversial. In PCPT, finasteride reduced the incidence of prostate cancer by 24.8%, compared to that with placebo. This reduction was almost exclusively for low-grade (Gleason score 6) tumors; an increased proportion of aggressive tumors (Gleason score ≥7) was observed.10 The REDUCE trial showed a relative risk reduction of 22.8% with dutasteride over a 4-year study period, as well as the detection of more high-grade tumors (Gleason score ≥8) in the dutasteride arm.29 The Combination of Avodart (dutasteride) and Tamsulosin (CombAT) trial also showed a 40% lower incidence of prostate cancer with dutasteride plus tamsulosin (an α-blocker agent), compared to that with tamsulosin alone. Unlike previous studies, the proportion of detected high-grade cancers (Gleason score ≥7) was not increased in the CombAT trial.13 In the present study, there were no differences between patients receiving 5ARI treatment and those who did not in the detection rates for prostate and high-grade cancers. Moreover, the use of 5ARIs was not associated with the prediction of high-grade cancer detection in multivariate analysis. In practice, the criteria of selecting patients for 5ARI treatment and the recommendation of repeat biopsy could be different.
Although the present study was retrospectively designed, our cohort reflected clinical practice: patients taking 5ARIs had lower PSA values and larger prostate volume than that of patients in the non-5ARI group. The cancer detection rate in patients with PSA levels of 4−10 ng/mL are 25% for the second biopsy and 24% for the third biopsy.30 The present cohort consisted of patients who underwent a second prostate biopsy, and the detection rate in patients taking 5ARIs (24.1%) was similar to that in previous studies. Furthermore, there was no difference between patients with and without 5ARI treatment in terms of the detection rate (p=0.747).
The present study has several limitations. First, the study was non-randomized, and certain data elements were obtained retrospectively. Therefore, heterogeneity was present in patients who underwent repeat prostate biopsy. Multiple factors, such as PSA kinetics, age, performance status, and patient compliance, affected clinical decision-making for the determination of the repeat biopsy. Second, although there is less chance of missing a cancer after performing two biopsies in 2 years, we do not know how many cancers were missed, as a lack of PSA increase was found in approximately 4% of cancers with a Gleason score of 7−10.29 Lastly, the cohort was relatively small. Thus, further studies with a larger number of patients are required to determine the clinical relevance of our findings.
In conclusion, the present study evaluated predictors for prostate cancer detection on a second prostate biopsy in patients with an initial negative biopsy who underwent 5ARI treatment. In practice, 5ARI treatment tended to be recommended for patients with a low PSA level and large prostate volume. The effects of 5ARIs on cancer detection and chemoprevention are beyond the scope of the present study. However, the present results suggest that repeat prostate biopsy should be considered for patients with more than two PSA increases after 6 months of 5ARI treatment.
References
1. Schröder FH. Landmarks in prostate cancer screening. BJU Int. 2012; 110(Suppl 1):3–7. PMID: 23046034.

2. Basch E, Oliver TK, Vickers A, Thompson I, Kantoff P, Parnes H, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol. 2012; 30:3020–3025. PMID: 22802323.

3. Presti JC Jr, O’Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003; 169:125–129. PMID: 12478119.

4. Koo KC, Lee DH, Lee SH, Chung BH. Peripheral zone prostate-specific antigen density: an effective parameter for prostate cancer prediction in men receiving 5α-reductase inhibitors. Prostate Int. 2013; 1:102–108. PMID: 24223410.

5. Choi YH, Cho SY, Cho IR. The different reduction rate of prostate-specific antigen in dutasteride and finasteride. Korean J Urol. 2010; 51:704–708. PMID: 21031091.

6. Aganovic D, Prcic A, Kulovac B, Hadziosmanovic O. Influence of the prostate volume, prostate specific antigen density and number of biopsy samples on prostate cancer detection. Med Arh. 2012; 66:41–44. PMID: 22482342.

7. Qi TY, Chen YQ, Jiang J, Zhu YK, Yao XH, Wang XJ. Utility of the transition zone index for identification of prostate cancer in Chinese men with intermediate PSA levels. Int Urol Nephrol. 2012; 44:807–815. PMID: 22311386.

8. Vickers AJ, Sjoberg DD. Decision analysis of dutasteride use for patients with negative prostate biopsy. Urology. 2015; 85:337–341. PMID: 25623680.

9. Oderda M, Zitella A, Richiardi L, Tizzani A, Gontero P. Effect of finasteride on the sensitivity of PSA to detect prostate cancer in rebiopsy series. Arch Ital Urol Androl. 2010; 82:135–138.
10. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349:215–224. PMID: 12824459.

11. Thompson IM Jr, Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013; 369:603–610. PMID: 23944298.

12. Pinsky PF, Black A, Grubb R, Crawford ED, Andriole G, Thompson I, et al. Projecting prostate cancer mortality in the PCPT and REDUCE chemoprevention trials. Cancer. 2013; 119:593–601. PMID: 22893105.

13. Roehrborn CG, Andriole GL, Wilson TH, Castro R, Rittmaster RS. Effect of dutasteride on prostate biopsy rates and the diagnosis of prostate cancer in men with lower urinary tract symptoms and enlarged prostates in the Combination of Avodart and Tamsulosin trial. Eur Urol. 2011; 59:244–249. PMID: 21093145.

14. Andriole GL, Guess HA, Epstein JI, Wise H, Kadmon D, Crawford ED, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998; 52:195–201. PMID: 9697781.
15. Redman MW, Tangen CM, Goodman PJ, Lucia MS, Coltman CA Jr, Thompson IM. Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach. Cancer Prev Res (Phila). 2008; 1:174–181. PMID: 19138953.

16. Kim SJ, Jeong TY, Yoo DS, Park J, Cho S, Kang SH, et al. Can prostate-specific antigen kinetics before prostate biopsy predict the malignant potential of prostate cancer. Yonsei Med J. 2015; 56:1492–1496. PMID: 26446628.

17. Celhay O, de la Taille A, Salomon L, Doré B, Irani J. Fluctuating prostate-specific antigen levels in patients with initial negative biopsy: should we be reassured. BJU Int. 2007; 99:1028–1030. PMID: 17324221.

18. Carter HB, Kettermann A, Ferrucci L, Landis P, Metter EJ. Prostate-specific antigen velocity risk count assessment: a new concept for detection of life-threatening prostate cancer during window of curability. Urology. 2007; 70:685–690. PMID: 17991538.

19. Ulmert D, Serio AM, O'Brien MF, Becker C, Eastham JA, Scardino PT, et al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008; 26:835–841. PMID: 18281654.

20. Marberger M, Freedland SJ, Andriole GL, Emberton M, Pettaway C, Montorsi F, et al. Usefulness of prostate-specific antigen (PSA) rise as a marker of prostate cancer in men treated with dutasteride: lessons from the REDUCE study. BJU Int. 2012; 109:1162–1169. PMID: 21699645.

21. van Leeuwen PJ, Kölble K, Huland H, Hambrock T, Barentsz J, Schröder FH. Prostate cancer detection and dutasteride: utility and limitations of prostate-specific antigen in men with previous negative biopsies. Eur Urol. 2011; 59:183–190. PMID: 21130560.

22. Lee KS, Koo KC, Cho KS, Lee SH, Han WK, Choi YD, et al. Indications for a second prostate biopsy in patients suspected with prostate cancer after an initial negative prostate biopsy. Prostate Int. 2017; 5:24–28. PMID: 28352620.

23. Andriole GL, Humphrey P, Ray P, Gleave ME, Trachtenberg J, Thomas LN, et al. Effect of the dual 5alpha-reductase inhibitor dutasteride on markers of tumor regression in prostate cancer. J Urol. 2004; 172:915–919. PMID: 15310997.
24. Andriole GL, Marberger M, Roehrborn CG. Clinical usefulness of serum prostate specific antigen for the detection of prostate cancer is preserved in men receiving the dual 5alpha-reductase inhibitor dutasteride. J Urol. 2006; 175:1657–1662. PMID: 16600723.
25. Brawer MK, Lin DW, Williford WO, Jones K, Lepor H. Effect of finasteride and/or terazosin on serum PSA: results of VA Cooperative Study #359. Prostate. 1999; 39:234–239. PMID: 10344212.

26. Kravchick S, Lobik L, Cytron S, Kravchenko Y, Dor DB, Peled R. Patients with persistently elevated PSA and negative results of TRUS-Biopsy: does 6-month treatment with dutasteride can indicate candidates for re-biopsy. what is the best of saturation schemes: transrectal or transperineal approach. Pathol Oncol Res. 2015; 21:985–989. PMID: 25753982.

27. van Houten ME, Gooren LJ. Differences in reproductive endocrinology between Asian men and Caucasian men--a literature review. Asian J Androl. 2000; 2:13–20. PMID: 11228931.
28. Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG. The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2006; 176:868–874. PMID: 16890642.
29. Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010; 362:1192–1202. PMID: 20357281.

30. Welch HG, Fisher ES, Gottlieb DJ, Barry MJ. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007; 99:1395–1400. PMID: 17848671.

Fig. 1
Algorithm to recommend repeat prostate biopsy in patients undergoing 5ARI treatment. 5ARIs, 5α-reductase inhibitors; PSA, prostate-specific antigen.
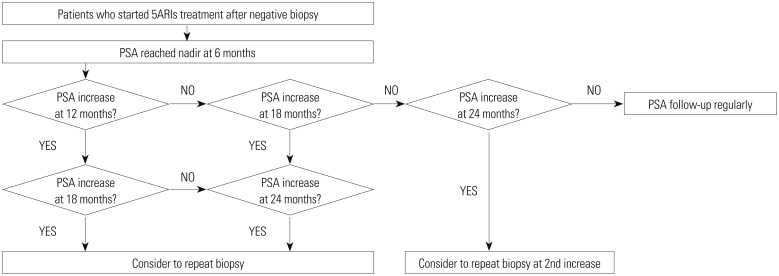
Table 1
Baseline Patient Characteristics
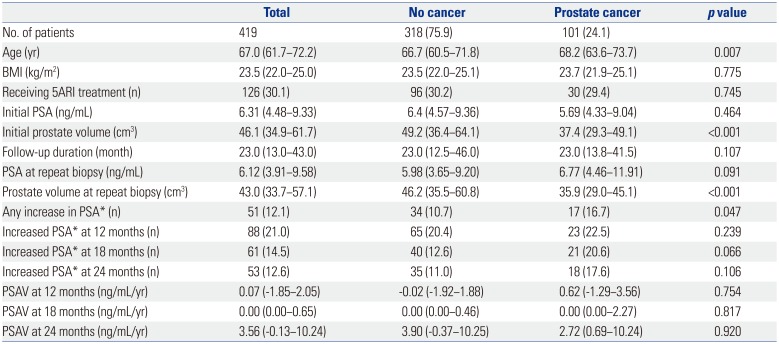
Table 2
Univariate and Multivariate Analyses for Predictive Factors of Prostate Cancer
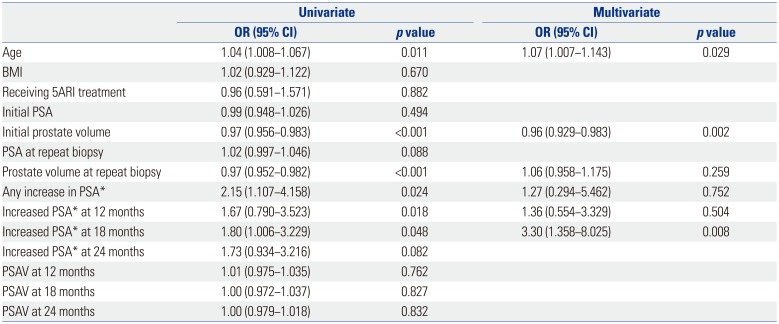
Table 3
Patient Characteristics According to the Use of 5ARIs
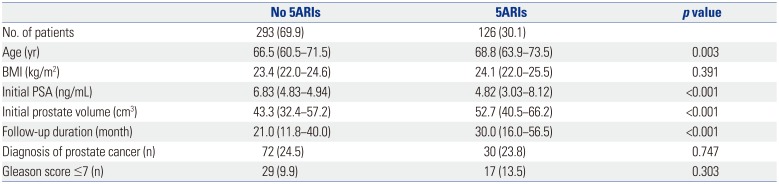
Table 4
PSA Level and Repeat Biopsy at 6-month Intervals According to the Use of 5ARIs
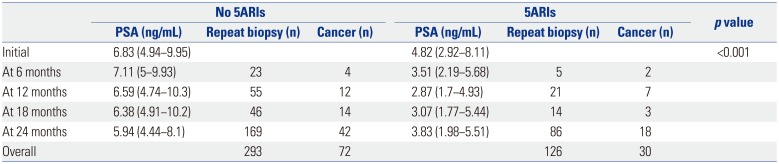
Table 5
Multivariate Analyses for Predictive Factors of Prostate Cancer According to the Use of 5ARIs
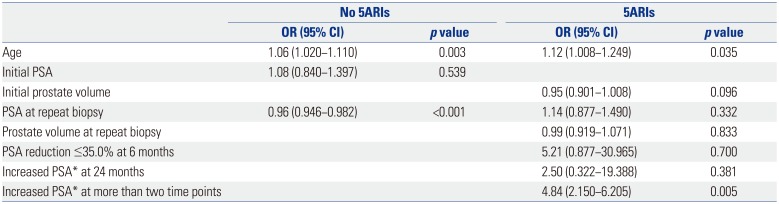
Table 6
Univariate and Multivariate Analysis for the Prediction of High-Grade Prostate Cancer (Gleason score ≥7)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download