Abstract
Purpose
Previous retrospective studies suggest that anaplastic lymphoma kinase (ALK) mutation-positive (ALK+) non-small cell lung cancer (NSCLC) patients are sensitive to pemetrexed. To determine its efficacy, we retrospectively evaluated clinical outcomes of pemetrexed-based chemotherapy in patients with ALK+ NSCLC.
Materials and Methods
We identified 126 patients with advanced, ALK+ NSCLC who received first-line cytotoxic chemotherapy. We compared response, progression-free survival (PFS), and overall survival (OS) rates according to chemotherapy regimens. Furthermore, we evaluated intracranial time to tumor progression (TTP) and proportion of ALK+ cells as prognostic factors.
Results
Forty-eight patients received pemetrexed-based chemotherapy, while 78 received other regimens as first-line treatment. The pemetrexed-based chemotherapy group showed superior overall response (44.7% vs. 14.3%, p<0.001) and disease control (85.1% vs. 62.3%, p=0.008) rates. The pemetrexed-based chemotherapy group also exhibited longer PFS (6.6 months vs. 3.8 months, p<0.001); OS rates were not significantly different. The lack of exposure to second-generation ALK inhibitors and intracranial metastasis on initial diagnosis were independent negative prognostic factors of OS. Intracranial TTP was similar between the treatment groups (32.7 months vs. 35.7 months, p=0.733). Patients who harbored a greater number of ALK+ tumor cells (≥70%) showed prolonged OS on univariate analysis (not reached vs. 44.8 months, p=0.041), but not on multivariate analysis (hazard ratio: 0.19, 95% confidence interval: 0.03–1.42; p=0.106).
The advent of tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) have produced notable improvements in treatment outcomes for patients with non-small cell lung cancer (NSCLC) who harbor genetic aberrancies corresponding to these proteins. Other genetic abnormalities, such as ROS1 or BRAF V600 mutations, are also reported to be targetable with specific TKIs.1234 Additionally, novel therapeutic strategies that target immune checkpoint proteins have also shown better efficacy than current standard chemotherapy regimens in patients advanced NSCLC with programmed death-ligand 1 expression.56
Crizotinib is the first TKI approved for the treatment of ALK mutation-positive (ALK+) NSCLC. In a large randomized phase III trial, more than half of the patients with ALK+ NSCLC achieved an objective response with a significant survival advantage over cytotoxic chemotherapy.2 However, most responders to crizotinib develop secondary resistance within one year. Although next-generation TKIs targeting ALK rearrangements, such as alectinib or ceritinib, have been shown to overcome resistance to crizotinib,78 patients with ALK+ NSCLC ultimately experience disease progression. Therefore, cytotoxic chemotherapy still plays an important role in the treatment of ALK+ NSCLC.
Pemetrexed is a cytotoxic chemotherapeutic agent that acts as an antimetabolite of folic acid. In a phase III trial, combination chemotherapy with pemetrexed and cisplatin showed non-inferiority in patients with advanced NSCLC, and showed superior outcomes, particularly in patients with non-squamous cell histology, compared to standard chemotherapy.9 Large subsequent studies consistently showed that pemetrexed exhibits significant activity against non-squamous NSCLC patients: it is now used frequently worldwide.10 Because most patients with ALK+ NSCLC have non-squamous histology, pemetrexed plays an important role in treatment, and some retrospective studies have shown that pemetrexed is more beneficial than other conventional cytotoxic chemotherapeutic agents in patients with ALK+ NSCLC111213 However, another study showed conflicting results;14 moreover, the original studies had important limitations, including heterogeneous populations and treatment strategies. Therefore, it remains unclear whether the clinical efficacy of pemetrexed-based chemotherapy is more favorable than that of other agents for patients with ALK+ NSCLC. The purpose of this study was to compare pemetrexed- and non-pemetrexed-based chemotherapy regimens in patients with advanced ALK+ NSCLC who received cytotoxic chemotherapy as first-line systemic treatment.
We enrolled patients with recurrent or unresectable ALK+ NSCLC who were treated between March 2008 and April 2015 at Seoul National University Bundang Hospital (Seongnam, Korea) and Seoul National University Hospital (Seoul, Korea). ALK positivity was defined as more than 15% of tumor cells exhibiting a split signal by break-apart fluorescence in situ hybridization (FISH) from among 50 or more analyzed tumor cells using the LSI ALK break-apart probe set (Vysis, Downers Grove, IL, USA).15
The inclusion criteria were 1) histologically confirmed recurrent or unresectable NSCLC; 2) receipt of cytotoxic chemotherapy in first-line systemic treatment after diagnosis with unresectable or recurrent NSCLC; and 3) adequate renal, hepatic, and bone marrow function. Patients who received ALK-directed therapy as a first-line systemic treatment were excluded.
The following data were retrospectively collected from electronic medical records: age, sex, smoking history, performance status, comorbidities, histologic characteristics [including immunohistochemistry (IHC) and molecular profiling], metastatic burden, disease status, chemotherapy regimen and response thereto according to the RECIST 1.1, progression-free survival (PFS), intracranial time to tumor progression (TTP), and overall survival (OS).16 Smoking history was classified as never, light, and heavy corresponding to ≤100 cigarettes in a lifetime, ≤10 pack-years, and >10 pack-years of smoking, respectively. Data were collected until February 2016.
The study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1607/354-102) and Seoul National University Hospital (IRB No. H-1606-103-771). The study was conducted according to the recommendations of the Declaration of Helsinki for biomedical research.
Continuous variables are expressed as means±standard deviations at baseline, while categorical variables are expressed as percentages. Student's t-test and one-way analysis of variance were used to analyze continuous variables, whereas the chi-squared or Fisher's exact test was used to analyze categorical variables. The Kaplan-Meier method and log-rank test were used to analyze differences in PFS, intra-cranial TTP, and OS depending on the clinical variables. After performing univariate analysis to identify potential prognostic factors, multivariate analysis was performed with Cox regression analysis using the enter method. The restricted cubic spline model that included the independent variables identified on Cox regression analysis was used to identify the proportion of ALK+ tumor cells and predict PFS and OS. The clinical impact of the proportion of ALK+ tumor cells on outcomes was evaluated by the log-rank test and Cox regression analysis. PFS was calculated as the interval between the first day of palliative chemotherapy and the day of documented disease progression or death of any cause; intracranial TTP was calculated as the interval between the first day of palliative chemotherapy and the first documentation of objective intracranial progression; and OS was calculated as the interval between the first day of palliative chemotherapy and the date of death. All p-values ≤0.05 were considered statistically significant. All statistical analyses were performed using SPSS software, version 21 for Windows (IBM Corp., Armonk, NY, USA).
In total, 126 patients were enrolled in this study, including 48 and 78 individuals who received pemetrexed- and non-pemetrexed-based chemotherapy in the first-line setting, respectively. The characteristics of patients in both groups are shown in Table 1 and Supplementary Table 1 (only online). The Eastern Cooperative Oncology Group performance status was 0 or 1 in 108 patients (93.1%). Almost all patients (124; 98.4%) had adenocarcinoma. Thirty-two patients (25.4%) had intracranial metastasis, while the lung was the most common site of metastasis (45.2%). IHC revealed positive expression of ALK in all 76 patients who were analyzed. Results of ALK FISH analyses were available for only 83 patients, as the remaining tissues were analyzed only at the central laboratory center during enrollment for the clinical trial. None of the patients harbored EGFR or KRAS mutations. Supplementary Table 2 (only online) shows the treatment outlines in the two groups. Gemcitabine plus either cisplatin or carboplatin was the most common combination regimen (49.2%). Overall, 116 (92.1%) and 43 (34.1%) patients were exposed to one or more ALK inhibitors and second-generation ALK inhibitors, respectively. There was no significant difference in the types of ALK inhibitor treatments between the two groups. Fifty (64.1%) patients were treated with pemetrexed-based chemotherapy during the follow-up period in the non-pemetrexed-based chemotherapy group. A total of 116 patients and 9 patients received platinum doublet chemotherapy and maintenance treatment, respectively. Sixteen patients in each group (33.3% and 20.5%, respectively) had intracranial metastasis on initial diagnosis, and more patients in the non-pemetrexed group tended to receive systemic therapy instead of local treatment for their lesions.
Table 2 shows the responses to first-line cytotoxic chemotherapy. The objective response rate (ORR) was superior in the pemetrexed group (44.7% vs. 14.3%, p<0.001), as was the disease control rate (85.1% vs. 62.3%, p=0.008). The median PFS was significantly longer in the pemetrexed group than in the non-pemetrexed group (6.6 months vs. 3.8 months, respectively; p<0.001) (Fig. 1). Factors related to PFS on univariate analysis (disease status, first-line platinum doublet chemotherapy, and first-line cytotoxic chemotherapy regimens) were subjected to multivariate analyses for PFS (Table 3). Non-pemetrexed based chemotherapy was the only independent prognostic factor for poorer PFS on multivariate analysis [hazard ratio (HR): 1.91; 95% confidence interval (CI): 1.27–2.87; p=0.002].
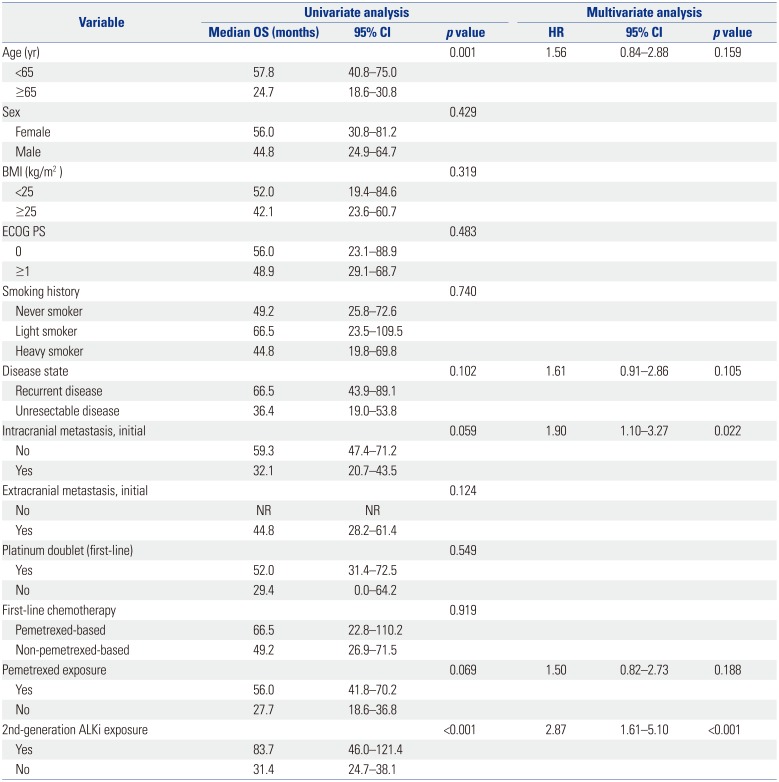
There was no significant difference in OS between the two groups (66.5 months vs. 49.2 months, p=0.919) (Fig. 2A). Exposure to pemetrexed was associated with a longer OS (56.0 months vs. 27.7 months, p=0.069) (Fig. 2B), although without statistical significance. However, OS was significantly longer in patients who received a second-generation ALK inhibitor (83.7 months vs. 31.4 months, p<0.001) (Fig. 2C). Factors, including age, disease status, initial intracranial metastasis, pemetrexed, and second-generation ALK TKI exposure, were subjected to multivariate analysis (Table 4), which showed that initial intracranial metastasis (HR: 1.90; 95% CI: 1.10–3.27; p=0.022) and non-exposure to second-generation ALK inhibitors (HR: 2.87; 95% CI: 1.61–5.10; p<0.001) were the only independent poor prognostic factors for OS.
Following analysis using the cubic spline model, ALK+ cell proportions of 35, 50, and 70% and of 35, 45, 55, and 70% were selected as candidate cut-off values for predicting PFS and OS, respectively. None of these candidate values were associated with PFS; however, a >70% proportion of ALK+ tumor cells was associated with significantly improved OS, compared to ≤70% ALK+ cells (not reached vs. 44.8 months, respectively; p=0.041) (Fig. 2D). However, there was no statistical significance for this cut-off value on multivariate analysis that included the presence of intracranial metastasis or exposure to a second-line ALK inhibitor (HR: 0.19, 95% CI: 0.03–1.42; p=0.106).
The median intracranial TTP was 32.7 months (95% CI: 19.8–45.6) (Supplementary Fig. 1A, only online), and was not different between the two groups regardless of initial intracranial metastasis status (Supplementary Fig. 1B-D, only online). In patients who had intracranial metastasis on initial diagnosis, the median intracranial TTP was longer in the pemetrexed group than in the non-pemetrexed group, although without statistical significance (30.5 vs. 6.3 months, respectively; p=0.242) (Supplementary Fig. 1D, only online).
We found that administering pemetrexed-based treatment as a first-line palliative cytotoxic chemotherapy is associated with better clinical outcomes in terms of ORR and PFS, but not with OS. Intracranial metastasis and exposure to second-generation TKIs were the only independent prognostic factors for OS. Pemetrexed-based chemotherapy is not associated with superior intracranial activity, compared to other chemotherapeutic agents. A high proportion of ALK+ tumor cells was also a possible prognostic factor for OS; however, multivariate analysis of the factor was not performed because detailed ALK FISH results were only available in a limited number of patients.
Previous retrospective studies have shown that pemetrexed-based chemotherapy is associated with a better tumor response and prolonged PFS in patients with ALK+ NSCLC, compared to other NSCLC subtypes.111213 Lee, et al.13 showed superior outcomes following pemetrexed single-agent treatment in patients with ALK+ NSCLC than in EGFR mutation-positive or wild-type NSCLC patients in second-line and subsequent settings, and also reported preclinical evidence of the antitumor activity of pemetrexed in ALK+ NSCLC. However, their study was a small retrospective study with heterogeneous patients and lines of treatment. Two other retrospective studies also included heterogeneous populations with different lines of treatment and did not directly compare pemetrexed-based chemotherapy for ALK+ NSCLC with others.1112 Most recently, Shaw, et al.14 compared the efficacy of pemetrexed and non-platinum/pemetrexed combinations in patients with ALK+ and ALK− NSCLC, and reported that the PFS rates of patients administered pemetrexed were similar regardless of ALK status. They concluded that only smoking status was predictive of clinical outcomes following pemetrexed-based chemotherapy. To the best of our knowledge, our study is the first to compare the efficacy of pemetrexed- vs. non-pemetrexed-based chemotherapy in patients with ALK+ NSCLC. Some preclinical and clinical data have shown that thymidylate synthase is a potential biomarker of pemetrexed treatment efficacy in patients with NSCLC and that levels of thymidylate synthase are lower in patients with ALK+ NSCLC.14171819 These findings may potentially explain the favorable outcomes for pemetrexed treatment in ALK+ NSCLC and our data. The benefit of pemetrexed may be related to its histologic subtype of adenocarcinoma rather than the molecular profile of ALK mutations. Almost all of the patients mentioned in the above studies had adenocarcinoma histology and pemetrexed showed a better efficacy in ALK+ NSCLC in comparison to NSCLC patients with other ALK mutation profiles.111213 Therefore, we suggest that the better efficacy of pemetrexed in these patients is related to the characteristics of NSCLC with ALK mutation itself.
Pemetrexed is the preferred regimen in patients with NSCLC adenocarcinoma based on the results of previous randomized studies.910 However, the superior outcomes of pemetrexed in such cancers were extrapolated from subgroup analyses in the JMDB trial, and there are limited data regarding direct comparisons of pemetrexed-based chemotherapy to other regimens, especially when analyzing OS.9 Pemetrexed has a favorable toxicity profile; therefore, some clinicians choose non-pemetrexed based regimens as first-line systemic treatments in a palliative setting if patients have good performance statuses, and reserve pemetrexed for subsequent treatments after disease progression when the patients' general conditions worsen. Although pemetrexed-based chemotherapy showed better tumor responses and prolonged PFS in our study, it was not associated with improved OS. Instead, the presence of intracranial metastasis and exposure to second-generation ALK inhibitors were independent predictive factors of OS.9 Based on our results, we cautiously recommend that pemetrexed may not necessarily be of use in a first-line setting, but that it can be utilized as a second- or subsequent-line therapy in patients with ALK+ NSCLC.
Although various ALK inhibitors are considered a standard in first-line or second-line treatment strategies for advanced ALK+ NSCLC, most patients experience acquired resistance to these agents. Therefore, it is clinically meaningful to evaluate the efficacy of cytotoxic chemotherapy before and after exposure to ALK inhibitors. A study by Berge, et al.20 reported that pemetrexed was active in either crizotinib-naïve (n=9, ORR=66%) or crizotinib-pretreated (n=4, ORR=75%) patients with ALK+ NSCLC. However, the size of the analyzed populations was too small to draw robust conclusions.
The overall incidence of intracranial metastasis is increasing, and its control is critical in ALK+ NSCLC.21 Crizotinib has limited central nervous system penetration, and the disparity between intracranial and extracranial responses presents a challenge in patients with ALK+ NSCLC.2223 Although second-generation ALK inhibitors show promising intracranial activity in patients with ALK+ NSCLC, cytotoxic chemotherapy still has an important role in these patients for the reasons mentioned above.724 Gandhi, et al.25 reported that high-dose pemetrexed in combination with high-dose crizotinib after intracranial progression occurs during standard dose crizotinib treatment shows positive intracranial responses, and some retrospective studies also showed that pemetrexed-based regimens exhibit potential intracranial activity.2627 In our study, pemetrexed-based chemotherapy was not associated with prolonged intracranial TTP; however, the study was a retrospective analysis and not all of the patients received scheduled brain imaging evaluations, except those who enrolled in clinical trials. Although regular brain imaging evaluation is usually performed when only clinically indicated in real practice, there may be many biases to evaluate intracranial response in our data. Therefore, further prospective studies are required to confirm or refute our results.
We found that ALK positivity in >70% of tumor cells is associated with prolonged OS on univariate analysis, but is not associated with PFS. Some studies found that the efficacy of crizotinib is correlated with the proportion of ALK+ tumor cells.2829 A possible explanation for these results is tumor heterogeneity, whose association with acquired resistance and poor response to treatment is well known in several types of cancers and has also been reported in ALK+ NSCLC.3031
Our study was a retrospective analysis; however, direct prospective comparison of pemetrexed to other agents in the first-line systemic palliative chemotherapy setting in patients with ALK+ NSCLC is currently impossible, since crizotinib is the treatment of choice in this setting. On the other hand, our study was limited to a population that received cytotoxic chemotherapy as first-line palliative chemotherapy; this relative homogeneity, as well as the relatively large number of patients, ought to render our results meaningful. The analysis of the impact of ALK+ tumor cell proportions on survival outcomes was limited to only some of our patients; therefore, we were able to find an association with OS only on univariate analysis, but not multivariate analysis. Because our study was a retrospective analysis, there may be potential selection bias. We could not use matching methods to overcome this limitation because patients with ALK+ NSCLC comprise a very small proportion of patients with NSCLC. Additional larger studies will be required to confirm our results.
A pemetrexed-based regimen may prolong PFS, compared to other regimens, in patients with ALK+ NSCLC when administered as first-line palliative chemotherapy. However, pemetrexed-based regimens did not exhibit better intracranial activity and were not associated with improved OS. Conversely, exposure to second-generation ALK inhibitors and no intracranial metastases on initial diagnosis were associated with improved OS in patients with ALK+ NSCLC. The proportion of ALK+ tumor cells may also be a possible clinical factor associated with OS.
Notes
References
1. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–246. PMID: 22285168.
2. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371:2167–2177. PMID: 25470694.

3. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015; 373:726–736. PMID: 26287849.

4. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014; 371:1963–1971. PMID: 25264305.

5. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639. PMID: 26412456.

6. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–135. PMID: 26028407.

7. Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016; 17:452–463. PMID: 26973324.

8. Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016; 34:661–668. PMID: 26598747.

9. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–3551. PMID: 18506025.

10. Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012; 13:247–255. PMID: 22341744.

11. Park S, Park TS, Choi CM, Lee DH, Kim SW, Lee JS, et al. Survival benefit of pemetrexed in lung adenocarcinoma patients with anaplastic lymphoma kinase gene rearrangements. Clin Lung Cancer. 2015; 16:e83–e89. PMID: 25682546.

12. Camidge DR, Kono SA, Lu X, Okuyama S, Barón AE, Oton AB, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011; 6:774–780. PMID: 21336183.

13. Lee JO, Kim TM, Lee SH, Kim DW, Kim S, Jeon YK, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011; 6:1474–1480. PMID: 21642865.

14. Shaw AT, Varghese AM, Solomon BJ, Costa DB, Novello S, Mino-Kenudson M, et al. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol. 2013; 24:59–66. PMID: 22887466.

15. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363:1693–1703. PMID: 20979469.
16. Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009; 45:261–267. PMID: 19091550.

17. Sun JM, Ahn JS, Jung SH, Sun J, Ha SY, Han J, et al. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin according to thymidylate synthase expression in nonsquamous non-small-cell lung cancer: a biomarker-stratified randomized phase II trial. J Clin Oncol. 2015; 33:2450–2456. PMID: 26124486.

18. Xu CW, Wang G, Wang WL, Gao WB, Han CJ, Gao JS, et al. Association between EML4-ALK fusion gene and thymidylate synthase mRNA expression in non-small cell lung cancer tissues. Exp Ther Med. 2015; 9:2151–2154. PMID: 26136951.

19. Takezawa K, Okamoto I, Okamoto W, Takeda M, Sakai K, Tsukioka S, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer. 2011; 104:1594–1601. PMID: 21487406.

20. Berge EM, Lu X, Maxson D, Barón AE, Gadgeel SM, Solomon BJ, et al. Clinical benefit from pemetrexed before and after crizotinib exposure and from crizotinib before and after pemetrexed exposure in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer. Clin Lung Cancer. 2013; 14:636–643. PMID: 23931899.

21. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015; 88:108–111. PMID: 25682925.
22. Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015; 33:1881–1888. PMID: 25624436.

23. Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011; 29:e443–e445. PMID: 21422405.

24. Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Gold KA, Rosell R, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016; 17:1683–1696. PMID: 27836716.
25. Gandhi L, Drappatz J, Ramaiya NH, Otterson GA. High-dose pemetrexed in combination with high-dose crizotinib for the treatment of refractory CNS metastases in ALK-rearranged non-small-cell lung cancer. J Thorac Oncol. 2013; 8:e3–e5. PMID: 23242445.

26. Moro-Sibilot D, Smit E, de Castro, Lesniewski-Kmak K, Aerts JG, Villatoro R, et al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: analysis from the European FRAME study. Lung Cancer. 2015; 90:427–432. PMID: 26791802.

27. Zhu W, Røe OD, Wu C, Li W, Guo R, Gu Y, et al. Activity of pemetrexed-based regimen as first-line chemotherapy for advanced non-small cell lung cancer with asymptomatic inoperable brain metastasis: a retrospective study. J Chemother. 2015; 27:221–226. PMID: 25735792.

28. Tanaka T, Yoshioka H, Haratani K, Hayashi H, Okamoto K, Kaneda T, et al. The association between the percentage of anaplastic lymphoma kinase(ALK)-positive cells and efficacy of ALK inhibitor (P3.02a-005). J Thorac Oncol. 2017; 12:S1162.
29. Lei YY, Yang JJ, Zhang XC, Zhong WZ, Zhou Q, Tu HY, et al. Anaplastic lymphoma kinase variants and the percentage of ALK-positive tumor cells and the efficacy of crizotinib in advanced NSCLC. Clin Lung Cancer. 2016; 17:223–231. PMID: 26454342.

30. Lee HJ, Seo AN, Kim EJ, Jang MH, Suh KJ, Ryu HS, et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol. 2014; 142:755–766. PMID: 25389328.

31. Zito Marino F, Liguori G, Aquino G, La Mantia E, Bosari S, Ferrero S, et al. Intratumor heterogeneity of ALK-rearrangements and homogeneity of EGFR-mutations in mixed lung adenocarcinoma. PLoS One. 2015; 10:e0139264. PMID: 26422230.

SUPPLEMENTARY MATERIALS
Supplementary Fig. 1
Overall intracranial TTP (A), as well as TTP, according to first-line cytotoxic treatment in all patients (B), negative intracranial disease status at initial presentation (C), and positive intracranial disease status at initial presentation (D). TTP, time to tumor progression; mIC, median intracranial; mo, months.
Fig. 1
PFS according to first-line cytotoxic treatment. PFS, progression-free survival; mPFS, median PFS; mo, months.
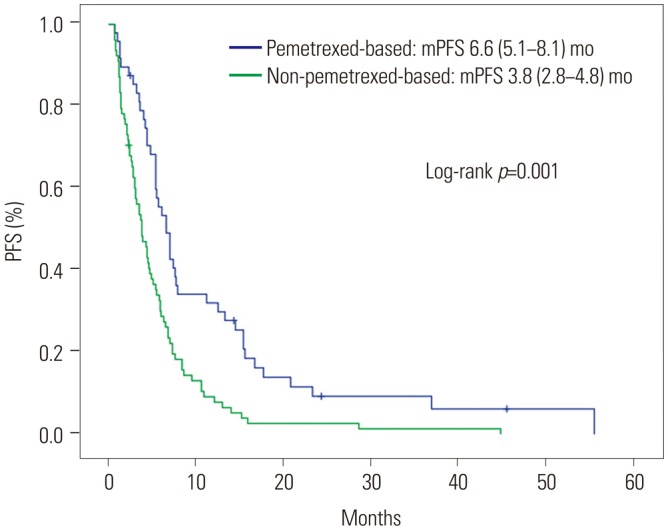
Fig. 2
OS according to first-line cytotoxic treatment (A), pemetrexed exposure (B), second-generation ALKi exposure (C), and ALK+ tumor cell proportion (D). OS, overall survival; ALK, anaplastic lymphoma kinase; FISH, fluorescence in situ hybridization; mOS, median overall survival; mo, months; ALKi, anaplastic lymphoma kinase inhibitor.

Table 1
Baseline Characteristics of Patients (n=126)
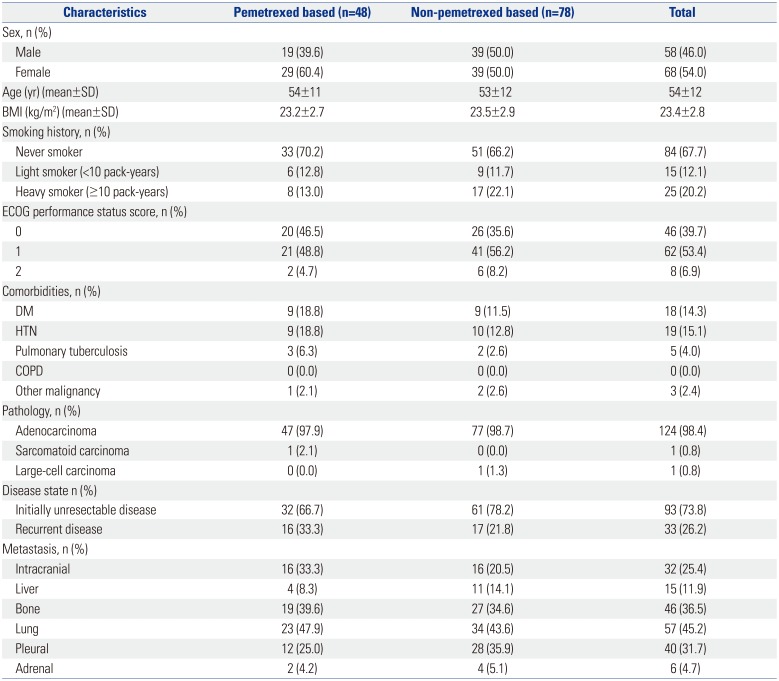
Table 2
Response to First-Line Cytotoxic Chemotherapy
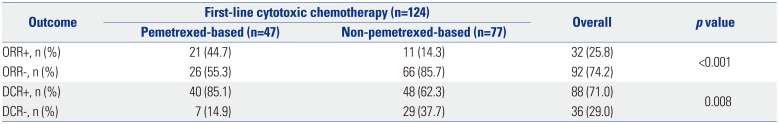
Table 3
Analysis of Prognostic Factors for PFS
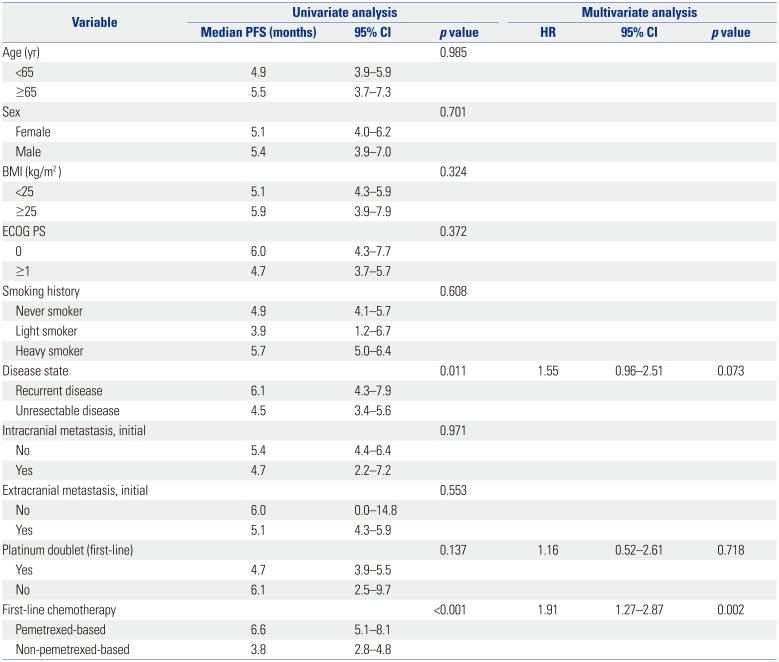
Table 4
Analysis of Prognostic Factors for OS




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download