This article has been
cited by other articles in ScienceCentral.
Abstract
The creation of transjugular intrahepatic portosystemic shunt (TIPS) is a widely performed technique to relieve portal hypertension, and to manage recurrent variceal bleeding and refractory ascites in patients where medical and/or endoscopic treatments have failed. However, portosystemic shunt creation can be challenging in the presence of chronic portal vein occlusion. In this case report, we describe a minimally invasive endovascular mesocaval shunt creation with transsplenic approach for the management of recurrent variceal bleeding in a portal hypertension patient with intra- and extrahepatic portal vein occlusion.
Go to :

Keywords: Portal hypertension, gastrointestinal hemorrhage, thrombosis, transjugular intrahepatic portosystemic shunt (TIPS), mesocaval, shunt
INTRODUCTION
Over the years, developments in the management of portal hypertension and variceal bleeding have led to a significant reduction in mortality.
1 Pharmacologic and endoscopic therapies remain the first-line treatment for prevention and treatment of variceal bleeding. In cases of first-line treatment failure, secondary options may be considered: surgical shunt creation, liver transplantation, and percutaneous treatments.
Transjugular intrahepatic portosystemic shunt (TIPS) is a well-described and widely performed technique to create a shunt between systemic and portal circulations, generally hepatic vein and portal vein, thereby bypassing the congested hepatic system. Unfortunately, portal vein thrombosis (PVT) occurs frequently in cirrhosis patients,
2 and have been reported to lower TIPS success rates to 40–75%.
3 While surgical shunt is a viable option, patients who do not meet surgical indications are left with very limited options. In this case report, we describe an endovascular mesocaval shunt creation with transsplenic approach to successfully manage variceal bleeding in a patient with total portal vein occlusion.
Go to :

CASE REPORT
A 16-year-old female patient with portal hypertension and chronic portal vein occlusion was admitted for persistent melena and severe anemia. Patient history included duodenum-preserving resection of pancreatic head and Roux-en-Y pancreaticojejunostomy due to choledochal cyst at early childhood. Capsule endoscopy confirmed active bleeding with a large amount of blood clot at proximal jejunum (
Fig. 1B). However, contrast-enhanced CT demonstrated total intra- and extrahepatic portal vein obliteration with intrahepatic collaterals and splenomegaly, rendering TIPS unfeasible (
Fig. 1A). Alternatively, mesocaval shunt formation was decided under informed consent. Under US-guidance, a direct puncture of the splenic vein was done using a 21-G Chiba needle (Cook Medical, Bloomington, IN, USA) and a 7-Fr Flexor Check-Flo Ansel modification sheath (Cook Medical). Angiography showed total obliteration of superior mesenteric vein (SMV) with cavernous transformation and collateral vessels (
Fig. 1C). An 11-mm Arrow balloon catheter (Teleflex, Dublin, Ireland) was placed at the SMV-splenic vein confluence as a fluoroscopic target. A 16-G Colapinto needle and a 9-Fr Teflon sheath (Transjugular Liver Access Set, Cook) were coaxially loaded over a guidewire via US-guided puncture of right internal jugular vein. Sheath tip was placed at the inferior vena cava (IVC), with a 0.035-inch guidewire placed at nearby common hepatic artery, due to concerns for vascular injury. Under fluoroscopic guidance, the Colapinto needle was advanced towards the target balloon, traversing the caudate lobe (
Fig. 2A and D). After successful puncture of SMV, predilation of the extrahepatic tract was performed with 5-mm Mustang balloon catheter (Boston Scientific, Marlborough, MA, USA). An 8-mm×7-cm stent-graft (S&G Biotech, Seongnam, Korea) was placed, followed by stent dilatation using an 8-mm FoxCross balloon catheter (Abbott Laboratories, Abbott Park, IL, USA). Another 7-mm×23-mm Palmas Blue stent (Cordis, Miami, FL, USA) was placed at splenic vein to prevent shunt wasting. Final angiography revealed patent flow between the SMV to IVC with an inverted “Y”-stent configuration (
Fig. 2B, C, and D) with decreased gastric varices. Portal vein pressure decreased immediately from 26-mmHg to 6-mmHg. Transsplenic tract embolization was done using histoacryl glue. No immediate complications occurred. At 9 month follow-up, mild gastrointestinal (GI) bleeding symptom recurred despite the disappearance of esophageal varices at endoscopy. Mild stenosis of the proximal stent was observed and treated with balloon dilatation. Shunt patency was maintained up to 24-month follow-up, with intermittent balloon dilatation of proximal stent stenosis.
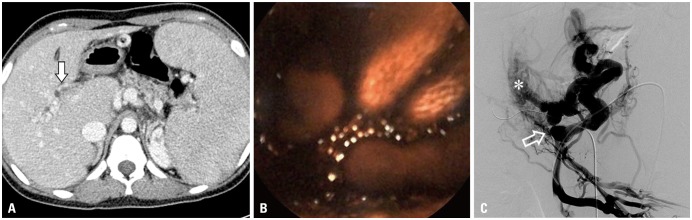 | Fig. 1Portal phase of preoperative contrast-enhanced CT image (A) shows total occlusion of portal vein due to extensive thrombosis (arrow) and cavernous malformation of intrahepatic portal veins with splenomegaly. Capsule endoscopy (B) revealed active bleeding and a large amount of blood clots at proximal jejunum. SMV angiography (C) shows cavernous transformation of intrahepatic portal vein (asterisk), and collateral vessels are seen. Total obliteration of SMV (open arrow) is noted. SMV, superior mesenteric vein.
|
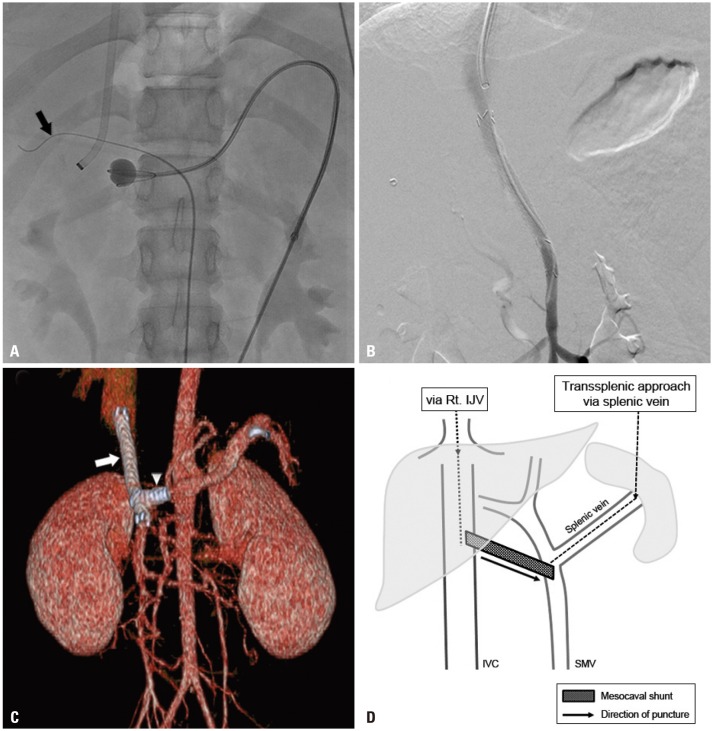 | Fig. 2Mesocaval shunt creation. (A) A 16-G Colapinto needle and a 9-Fr Teflon sheath were coaxially loaded over a guidewire via US-guided puncture of right IJV. Via US-guided puncture of the splenic vein, an 11-mm balloon catheter was placed at the SMV-splenic vein confluence. The inflated balloon was used as a fluoroscopic target. A 0.035-inch guidewire (black arrow) was placed in the dilated hepatic artery to avoid puncture of the target. (B) Final angiography shows patent mesocaval shunt between IVC and SMV. (C) CT-rendered three-dimensional image reveals patent stent graft (white arrow) with partial intrahepatic portion at the proximal end. Additional stent at distal splenic vein (arrowhead) to prevent shunt wasting resulted in an inverted “Y” configuration of stents. (D) Simplified diagram of transsplenic mesocaval shunt technique. Linear arrow represents the direction of puncture (splenic vein stent is not shown). IJV, internal jugular vein; SMV, superior mesenteric vein; IVC, inferior vena cava.
|
Go to :

DISCUSSION
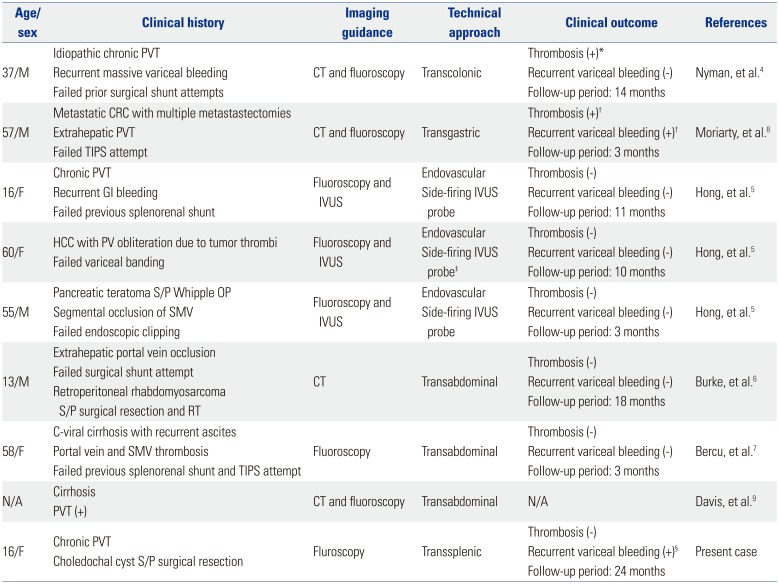
Initially described by Nyman, et al.,
4 a total of eight cases of mesocaval shunt creation in the presence of chronic portal occlusion
45678 have been reported in English publications (
Table 1). While mesocaval shunt creation may be offered to patients who are not surgical and/or TIPS candidates, certain anatomic requirements must be met. Preoperative contrast-enhanced CT should be performed to evaluate anatomic feasibilities, since potential complications may vary depending on the patient's anatomy.
9 Injuries may occur at adjacent vasculatures, pancreas, duodenum, and any other structures. One case reported mesocaval shunt creation through the pancreatic uncinate process without complications.
5 However, transpancreatic approach involves theoretical complications of pancreas bisection leading to pancreatic hemorrhage or inflammation
7 and should be avoided if possible.
Table 1
Summary of Previous Case Reports of Mesocaval Shunt Creation
|
Age/sex |
Clinical history |
Imaging guidance |
Technical approach |
Clinical outcome |
References |
|
37/M |
Idiopathic chronic PVT |
CT and fluoroscopy |
Transcolonic |
Thrombosis (+)*
|
Nyman, et al.4
|
|
Recurrent massive variceal bleeding |
Recurrent variceal bleeding (−) |
|
Failed prior surgical shunt attempts |
Follow-up period: 14 months |
|
57/M |
Metastatic CRC with multiple metastastectomies |
CT and fluoroscopy |
Transgastric |
Thrombosis (+)†
|
Moriarty, et al.8
|
|
Extrahepatic PVT |
Recurrent variceal bleeding (+)†
|
|
Failed TIPS attempt |
Follow-up period: 3 months |
|
16/F |
Chronic PVT |
Fluoroscopy and IVUS |
Endovascular |
Thrombosis (−) |
Hong, et al.5
|
|
Recurrent GI bleeding |
Side-firing IVUS probe |
Recurrent variceal bleeding (−) |
|
Failed previous splenorenal shunt |
Follow-up period: 11 months |
|
60/F |
HCC with PV obliteration due to tumor thrombi |
Fluoroscopy and IVUS |
Endovascular |
Thrombosis (−) |
Hong, et al.5
|
|
Failed variceal banding |
Side-firing IVUS probe‡
|
Recurrent variceal bleeding (−) |
|
Follow-up period: 10 months |
|
55/M |
Pancreatic teratoma S/P Whipple OP |
Fluoroscopy and IVUS |
Endovascular |
Thrombosis (−) |
Hong, et al.5
|
|
Segmental occlusion of SMV |
Side-firing IVUS probe |
Recurrent variceal bleeding (−) |
|
Failed endoscopic clipping |
Follow-up period: 3 months |
|
13/M |
Extrahepatic portal vein occlusion |
CT |
Transabdominal |
Thrombosis (−) |
Burke, et al.6
|
|
Failed surgical shunt attempt |
Recurrent variceal bleeding (−) |
|
Retroperitoneal rhabdomyosarcoma S/P surgical resection and RT |
Follow-up period: 18 months |
|
58/F |
C-viral cirrhosis with recurrent ascites |
Fluoroscopy |
Transabdominal |
Thrombosis (−) |
Bercu, et al.7
|
|
Portal vein and SMV thrombosis |
Recurrent variceal bleeding (−) |
|
Failed previous splenorenal shunt and TIPS attempt |
Follow-up period: 3 months |
|
N/A |
Cirrhosis |
CT and fluoroscopy |
Transabdominal |
N/A |
Davis, et al.9
|
|
PVT (+) |
|
16/F |
Chronic PVT |
Fluroscopy |
Transsplenic |
Thrombosis (−) |
Present case |
|
Choledochal cyst S/P surgical resection |
Recurrent variceal bleeding (+)§
|
|
Follow-up period: 24 months |

While most of the reported cases created entirely extrahepatic mesocaval shunts, we created a partially intrahepatic shunt by traversing the caudate lobe. A potential advantage for partially intrahepatic shunt is the prevention of shunt migration. Because a completely extrahepatic shunt lacks suitable anchoring structures, acute or delayed shunt migration has been suggested as a potential complication.
5 It should be noted that, in the presented case, mild stenosis without thrombotic occlusion of the stent graft required repeated balloon dilatation. Whether the surrounding hepatic parenchyme contributed to intrahepatic stent graft stenosis is unclear.
To our best knowledge, this is only the second case of endovascular mesocaval shunt creation, with a unique use of transsplenic approach. Transcolonic,
4 transgastric,
8 or transabdominal
679 routes have been utilized, but there are concerns for increased risks of peritonitis, sepsis and hemorrhage.
5689 Alternatively, a direct puncture of the splenic vein under US-guidance was adopted. Although not widely advocated, transsplenic approach has been shown to be safe and feasible for portal vein intervention with preventable complications.
10111213 Moreover, transsplenic endovascular process can bypass direct puncture of the abdominal wall, intestines, and major vessels with a single needle, reducing the risk of infection. In addition, the risk of hemorrhage may be reduced since only one wall of the SMV is punctured.
5
Despite the lack of long-term mortality analysis, mesocaval shunt offers an effective alternative treatment in patients with limited options due to PVT. Previous reports indicate that patients did not have recurrent thrombosis or variceal bleeding for as long as 18 months. Although our patient developed recurrent GI bleeding at 9 months, mesocaval shunt remained patent up to 24 months, which is the longest follow-up period up to date. Therefore, mesocaval shunt offers an alternative treatment in cases where TIPS cannot be applied. To determine its utility in clinical settings, further research on long-term durability of mesocaval shunt is warranted.
In conclusion, intrahepatic and extrahepatic PVT or occlusion poses a great challenge in creating percutaneous portosystemic shunts in portal hypertension patients. We present a successful fluoroscopy-guided, endovascular mesocaval shunt creation technique that can relieve portal hypertension and variceal bleeding in patients with total portal vein occlusion.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download