INTRODUCTION
Mitochondrial disease (MD) is a hereditary or sporadic multisystemic disorder resulting from the impairment of mitochondrial energy metabolism in the respiratory chain complex of the mitochondrial inner membrane.
12 The heart is one of the most commonly affected organs, because the myocardium requires a high level of oxygen metabolism to supply blood and energy substrates.
123 In addition, mitochondria have a significant role in the energy production of cardiac cellular biogenic arrangements. Energy supplied in the form of adenosine triphosphate (ATP) is essential in sustaining cardiac contractility and relaxation functions.
MD can result in structural heart lesions, which may affect the myocardium, coronary arteries, pericardium, or aortic root. Cardiovascular functional abnormalities can also occur, including impulse generation, conduction abnormalities, systolic dysfunction, heart failure, pulmonary hypertension, and autonomic dysfunction.
1 The most frequent cardiovascular manifestation is cardiomyopathy (CMP), which presents as hypertrophic CMP (HCMP), dilated CMP, restrictive CMP, or unclassified CMP resembling left ventricular hypertrabeculation or noncompaction. Myocardial fibrosis and late enhancement are also present in other cardiomyopathies.
123
Children and infants with MD have different clinical presentations to people with adult-onset MD. In children and infants, MD is often correlated with genetic defects. The phenotypes of patients with pediatric MD are much more severe, often involving the brain and multi-systemic disorders, but seldom isolated myopathy. The frequency of cardiovascular involvement may differ between children and adults.
With the above in mind, the purpose of this study was to investigate cardiovascular function in children with MD, to assess conventional and advanced echocardiographic data, to distinguish unique myocardial characteristics, and to identify early myocardial deterioration.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the medical records of 33 children with MD who were treated at Gangnam Severance Hospital between March 2013 and June 2014. An equal number of healthy, age-matched children were enrolled to serve as a control group.
All patients with MD satisfying the modified MD criteria proposed by Bernier, et al.
4 were enrolled; mitochondrial respiratory chain complex defects were confirmed via muscle tissue biochemical enzyme assay. The mitochondrial enzyme function of isolated mitochondria was assessed using a standard spectrophotometric assay as described by Rustin, et al.
5 Nicotinamide adenine dinucleotide, coenzyme Q (CoQ) reductase (complex I), succinate-CoQ reductase (complex II), succinate-cytochrome c reductase (complex II–III), cytochrome c reductase (complex III), cytochrome c oxidase (complex IV), oligomycin-sensitive ATPase (complex V), and citrate synthase activity were also evaluated. Patients were diagnosed with mitochondrial respiratory chain complex defects when the residual enzyme activity was <20% of that in the age-matched control group.
The demographic characteristics of patients were collected from their medical records. The control group of children comprised those from echocardiographic referral for precordial cardiac murmur, an enlarged cardiothymic silhouette in a chest radiogram, or an incomplete right bundle branch block pattern on an electrocardiogram. Children with any acute illness by history and physical examination, congenital or acquired heart disease, syndromes, or chromosomal abnormalities were excluded.
All study participants underwent transthoracic echocardiography. All procedures were approved by the Institutional Review Board of Gangnam Severance Hospital in Seoul, Korea (3-2017-0168). Informed consent was obtained and all methods were performed in accordance with the relevant guidelines and ethics board regulations.
Echocardiography and strain analysis
Conventional echocardiographic measurements and advanced myocardial imaging studies were performed by an expert pediatric cardiologist in both groups using a Siemens ACUSON SC2000 system (Siemens Medical Solutions USA, Inc., Mountain View, CA, USA). Echocardiography was performed in the left lateral decubitus or supine position to obtain two-dimensional, M-mode, and Doppler measurements of the parasternal long-axis view, short-axis view, and four-chamber view, respectively. Maximal left ventricular wall thickness was defined as the largest thickness in any single segment, mainly in the parasternal short-axis view. The mean value of more than three measurements obtained using echocardiography was entered into analysis.
Advanced tissue speckle images were recorded at a rate of >50 frames/s to investigate myocardial strain and the rate thereof. Two-dimensional images of the apical four-chamber view were recorded to assess longitudinal strain, while those of the parasternal short-axis view were acquired to assess radial and circumferential strain. An offline speckle tracking process was conducted with acoustic markers using Syngo Velocity Vector Imaging software (Siemens Medical Solution USA, Inc.). The borders of the endocardium and epicardium were traced manually and the border of the myocardium was defined as the midline between the border of the endocardium and epicardium. These three contours were tracked frame-by-frame throughout the cardiovascular cycle. Patients were diagnosed with myocardial deformation after this tracking process, while simultaneously checking for layer-specific myocardial strain and the respective strain rate of the layers of the endocardium, myocardium, and epicardium.
Statistical analysis
All data and measurements were reported as means±standard deviations. We compared all demographic and echocardiographic data between the two groups using an independent Student's t-test. A p-value ≤0.05 was considered statistically significant with a correlation r-value of ≥0.60 with SPSS 19.0 (IBM Corp., Armonk, NY, USA).
RESULTS
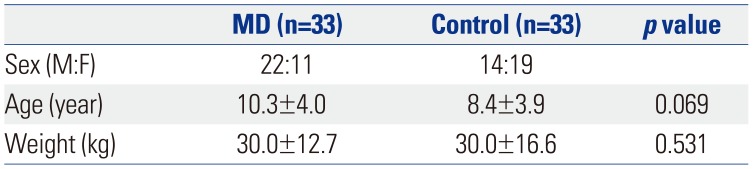
In total, 22 boys and 11 girls with a mean age of 10.3±4.0 years were included in the analysis. The control group included the same number of children (14 boys and 19 girls) with a mean age of 8.4±3.9 years (
Table 1). The results of the spectrophotometric biochemical enzyme assays for mitochondrial respiratory chain complexes of the muscle were available in all patients: 66.7% (n=22) of the patients had complex I defects and 33.3% (n=11) had complex IV defects. Among the children with MD, 10 were capable of independent walking, eight were wheelchair-bound, and 15 were bed ridden.
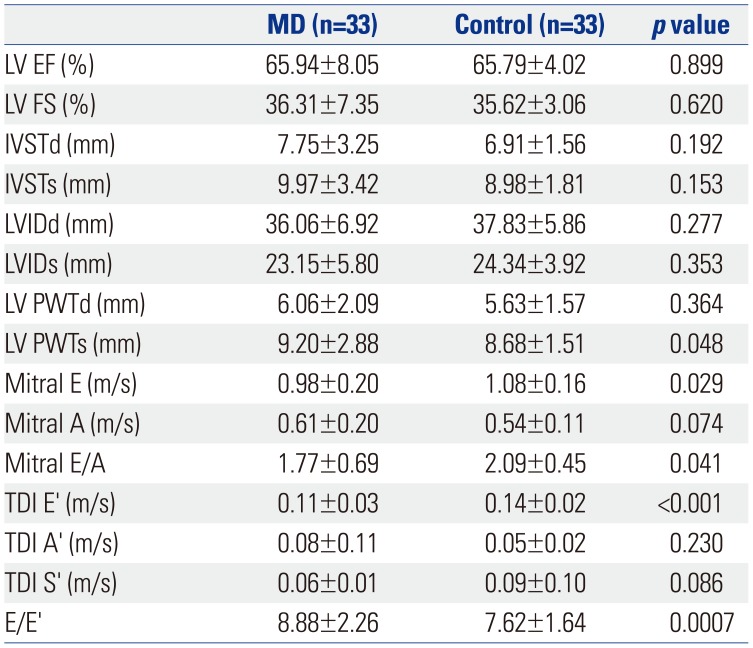
The conventional echocardiographic measurements of left ventricular ejection fraction were similar between the two groups [MD, 65.8±9.8%; control, 65.9±4.2%;
p=not significant (ns)]. The results of fractional shortening were also similar between groups (MD, 35.6±6.3%; control, 36.3±3.2%;
p=ns). Patients with MD had greater interventricular septal and left ventricular posterior wall thickness during diastole and systole than patients in the control group. However, this difference was not statistically significant (
p=ns) (
Table 2).
The conventional mitral inflow E, E/A, tissue Doppler diastolic E' were significantly lower, and E/E' was higher in the MD group than those in the control group (
p<0.05) (
Table 2). These diastolic parameters did not correlate with wall thickness in this study.
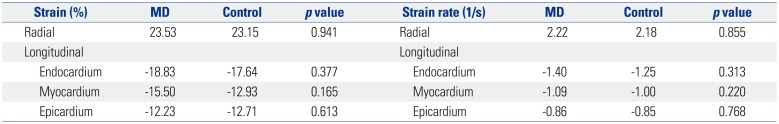
We noted no difference in longitudinal strain in the apical four-chamber view between the two groups in all layers of the endocardium, myocardium, and epicardium. Strain rate was not significantly different between the two groups in the apical four-chamber view (
Table 3).
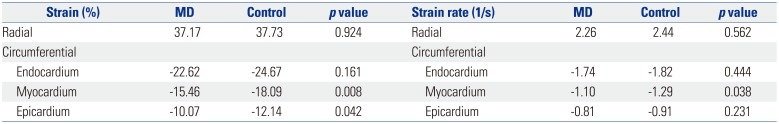
Radial strain measured in the parasternal short-axis view was not statistically different between the groups (MD, 37.17±11.1%; control, 37.73±25.9%; p=0.924). Similarly, the radial strain rate was not significantly different between the groups (MD, 2.26±0.91; control, 2.44±0.84; p=0.562).
Circumferential strain in the endocardium (-22.62±4.7% vs. -24.67±5.3%;
p=0.161), myocardium (-15.46±3.8% vs. -18.09±4.0%;
p=0.008), and epicardium (-10.07±4.0% vs. -12.14±5.0%;
p=0.042) was lower in patients with MD than in healthy patients. Circumferential strain rate of the myocardium was also lower in the MD group (-1.10±0.27) than that in the control group (-1.29±0.22) (
p=0.038). The strain rate was lower in the endocardium (-1.74±0.38 vs. -1.82±0.27;
p=0.444) and epicardium (-0.81±0.38 vs. -0.91±0.27,
p=0.231) of patients in the MD group than in patients in the control group, although the difference was not statistically significant (
Table 4). Interestingly, the decreased circumferential strain of the endocardium, myo-cardium, and epicardium in patients with MD correlated well with the tissue Doppler peak velocities of E′ (
Figs. 1,
2, and
3).
When these results were considered together, we found that the conventional echocardiographic diastolic values of mitral E, E/A, and tissue Doppler E′ were significantly lower in children with MD than those in healthy children. In contrast, E/E′ was significantly higher in children with MD than that in he-althy children. The advanced myocardial analysis revealed no significant differences in longitudinal and radial strain between the two study groups; however, the circumferential strain in patients with MD was significantly lower in the myocardium and epicardium. In addition, the circumferential strain in all three layers correlated with tissue Doppler E′.
DISCUSSION
Hundreds of different pathogenic mitochondrial DNA mutations have been reported in humans, many of which are associated with various cardiovascular diseases. This wide range of disease expression and the lack of a specific cardiovascular phenotype distinctive to patients with MD presents a challenge for cardiologists, especially in pediatric patients.
6 According to previously published studies, cardiac involvement was reported in 33% of pediatric patients with MD, while CMP was present in approximately 5.6% of children with MD.
178
Among the different types of CMP, HCMP is the most frequently observed in patients with MD.
1910 Cardiac hypertrophy is an adaptive response to the increased work load induced by physiological or pathological stimuli to counteract the increased wall tension and to maintain cardiac output.
11 When the heart is extremely stressed with persistent overload, the resulting hypertrophy may become maladaptive, and cardiac function may progressively deteriorate, resulting in heart failure.
12
Primary mitochondrial cardiomyopathies lead to mitochondrial proliferation in cardiomyocytes.
13 In addition, pathological cardiac hypertrophy that develops due to increasing numbers of mitochondria results in the enhancement of mitochon-drial biogenesis and protein synthesis.
14 To our knowledge, however, the implications of mitochondrial biogenesis in CMP have not been clarified. Furthermore, the hypertrophic phase induced in CMP that naturally accompanies increased mitochondrial biogenesis seems to delay the cardiac decompensation caused by pressure overload.
15
Other experimental studies have reported the cardiovascular effects of prediabetes in patients with MD, indicating that mild diastolic dysfunction and cardiovascular hypertrophy are multifactorial phenomena associated with early changes in mitophagy, cardiac lipid accumulation, and elevated oxidative stress.
16 Prediabetes-induced oxidative stress was also shown to originate from the subsarcolemmal mitochondria.
16
Severe and rapidly progressive dilated CMP has been shown to occur if the hypertrophic phase is bypassed.
17 Since cardiovascular systolic and diastolic function is dependent on mitochondrial ATP, it is possible that a decline in mitochondrial biogenesis contributes to the progression of CMP and heart failure, leading to sudden cardiovascular death.
Many questions still remain to be answered, however. The reason why CMP is the most common form of cardiac involvement in MD may be due to the close relationship between myocardial function and mitochondrial energy supply. Additionally, the similarities between skeletal muscle and the myocardium may play a role, with the former being the most frequently affected organ due to its need for constant energy supply and the latter being the tissue with potentially the greatest energy requirement.
1 HCMP is most prevalent in children with MD and develops even without the presence of systolic dysfunction or heart failure.
8
In the present study, we identified an increase in the left ventricular posterior wall and interventricular septal thickness, which may indicate the beginning of myocardial hypertrophy preceding HCMP. Furthermore, the diastolic measurement of mitral inflow, E, E/A, tissue Doppler E′, and E/E′ were all significantly different, suggesting that statistically significant diastolic dysfunction had already developed. This diastolic deterioration certainly seems to have preceded the hypertrophic change of the myocardium. The decline of diastolic function could represent the beginning of real myocardial deterioration. Pathological hypertrophy may then progress in the interventricular septum and ventricular posterior free wall along with mitochondrial biogenesis to overcome the persistent lack of sufficient energy to meet the workload demanded.
To diagnose patients with early cardiac deterioration, diverse echocardiographic techniques should be used in patients with MD, along with conventional and advanced myocardial imaging studies. Myocardial layer-specific strain analysis is a feasible and reproducible method to assess subtle myocardial deterioration.
18 However, to our knowledge, it has rarely been used in children. In this study, children with MD demonstrated decreased diastolic parameters, compared to those in healthy controls. We also tried to perform layer-specific myocardial analysis of the endocardium, myocardium, and epicardium in this patient population for the first time. Interestingly, we observed circumferential strain deterioration in the myocardium and epicardium despite preserved longitudinal and radial strain with normal left ventricular ejection fraction.
Earlier deterioration of circumferential strain may be due to the configuration of left ventricular myofibril derangement in patients with MD. As myocardial fiber arrangement is important for electrical propagation and myocardial contraction, once subtle myofibril dysfunction develops, the conduction problem would be unavoidable. Moreover, myofibril dysfunction is the obvious cause for further contractile dysfunction. In other words, decreased circumferential strain could serve as an early sign of myocardial dysfunction before it is severe en-ough to produce clinical detectable changes and while other conventional systolic parameters are still within reasonable ranges. Therefore, circumferential strain can be a sensitive indicator of myocardial deterioration before the development of myocardial fibrosis and the subsequent loss of myocytes.
Among the three layers of the myocardium, we recognized that, in terms of order and severity, circumferential strain alteration started from the mid-myocardial layer and progressed to the epicardial layer. The endocardial layer may be less deteriorated or relatively preserved in terms of functional decline, compared with the middle myocardium and epicardium. This could indeed be a promising valuable discovery in relation to myocardial assessment in pediatric patients with MD. Furthermore, circumferential strain in all three layers correlated well with early diastolic tissue Doppler velocity of E′, which may imply systolic and diastolic coupling in these children.
The goal of cardiovascular treatment is to decelerate the deterioration of myocardial function with appropriate drugs to allow a better quality of life, regardless of systemic multi-organ involvement. Even in patients showing values in the normal ranges for various parameters of conventional echocardiography, advanced detailed functional indicators, such as strain measurements, may help to recognize early signs of dysfunction, as demonstrated in this study.
Myocardial strain analysis of the respective cardiac layers is certainly a promising tool with which to detect subtle myocardial deterioration before any noticeable decline of cardiac function occurs, aiding in determining when to proceed with suitable cardiac treatment. It could undoubtedly play a role in the enhancement of cardiac function and thus improve prognosis with respect to growth and development in children with MD. The limitation of this study is a small sample size of the rare disease children with MD. Further studies are needed to provide a better and more comprehensive investigation.
In conclusion, children with MD may have earlier myocardial deterioration in circumferential strain, especially of the middle myocardial and epicardial layers, even with well-preserved longitudinal function. This knowledge could be used as an early diagnostic indicator of myocardial involvement, aiding in diagnosis and allowing for timely treatment of CMP.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download