INTRODUCTION
Kawasaki disease (KD) is an acute systemic vasculitis that primarily affects children younger than 5 years of age. There are no specific laboratory tests for the diagnosis of KD; therefore, diagnosis is usually made according to clinical criteria.
1 Following Japan, Korea has the second highest prevalence of KD in the world, and the incidence of this disease is increasing steadily.
2 Although KD is a self-limiting disease, if not treated properly with intravenous immune globulin (IVIG), coronary artery lesions (CALs) can occur. In developed countries, KD is the most common cause of acquired heart disease.
KD exhibits an epidemic pattern. The peak incidence is at 9−11 months of age, coinciding with waning maternal immunity. Symptoms are similar to those of other infectious diseases, and the course of disease is usually self-limited. For these reasons, KD was once thought to be an infectious disease. Several organisms were reported as causative agents, including viruses and species of some bacteria, such as
Streptococcus and
Staphylococcus. However, no causative infectious organisms have been isolated from patients.
3
Genetic predisposition to KD has also been suggested. The annual incidence of KD worldwide is highest and is increasing in Japan, Korea, and Taiwan. This incidence is 10−20 times higher than that of Western countries. Interestingly, the same incidence level in people of Japanese ancestry living in Hawaii indicates that the predilection for Asian populations may not be due solely to geographic factors. KD also exhibits familial disposition. The relative risk for siblings is about 10-fold greater, and a recent study revealed that two-generation KD patients is more prevalent than expected. HLA types and allotypes of immunoglobulin in relation to KD have been studied, although results differ by study and region. Lack of agreement between studies may be due to differences in genetic background among the different races involved. Thus, KD can be thought of as a complex multifactorial disease that develops in association with unidentified infectious organisms in children with a predisposing genetic background.
4
With the recent advent of genome-wide association studies, remarkable progress has been made in identifying candidate genes associated with KD.
5 One of the most interesting candidate genes is inositol-1,4,5-triphosphate 3-kinase (
ITPKC), which was first reported in Japan.
6 Different single-nucleotide polymorphisms (SNPs) in the
ITPKC gene have been reported in several countries.
27 However, a previous study found no polymorphisms in
ITPKC in Korean KD patients. Interestingly, a recent study reported that polymorphisms in
ITPKC are associated with reactivation of Bacille Calmette-Guérin (BCG) injection scars.
8
Erythema and induration of the BCG injection site is a specific clinical feature of KD
910 and is present in approximately 30−50% of KD patients, especially in children who are younger than 2 years of age.
111213 Although erythematous changes in the BCG inoculation site in patients with human herpes virus type 6 infection have been reported,
14 this clinical feature is known as a specific finding of KD. Chun, et al.
15 used BCG in the development of an animal model of KD in programmed death-1 gene knockout mice. These findings suggest a possible correlation between BCG and the pathogenesis of KD.
In inbred mice, resistance and susceptibility to the growth of BCG is controlled by the BCG locus, also known as the natural resistance-associated macrophage protein 1 (
NRAMP1) gene. The
NRAMP1 gene was later renamed solute carrier 11a1 (
SLC11A1), and the human homologue was subsequently isolated. Interactions of macrophages with bacterial lipopolysaccharide and/or natural killer cell−or T cell−derived interferon-γ are regulated by
SLC11A1. Polymorphisms in
SCL11A1 are thought to induce a hypersensitivity reaction to the BCG strain. A relationship between
SCL11A1 polymorphisms and KD has been reported.
16
This study examined polymorphisms in the
ITPCK gene in Korean patients with KD. Polymorphisms in
SLC11A1 were also examined to determine a possible association with KD. The relationship between KD susceptibility and polymorphisms in these genes was also examined. As other studies have investigated interactions between these two genes, especially with respect to synergistic effects,
1718 gene-gene interactions and/or synergistic effects between
SCL11A1 and
ITPKC polymorphisms in KD were also investigated.
Go to :

DISCUSSION
The KD-associated gene
ITPKC was first identified and studied in Japan.
6 Many studies have shown relatively higher frequencies of
ITPKC in Japanese (0.16) and European populations (0.15), compared with Taiwanese populations (0.054).
7 Consistent with geographic differences in SNPs, one study conducted in Korea did not find similar polymorphisms in
ITPKC.
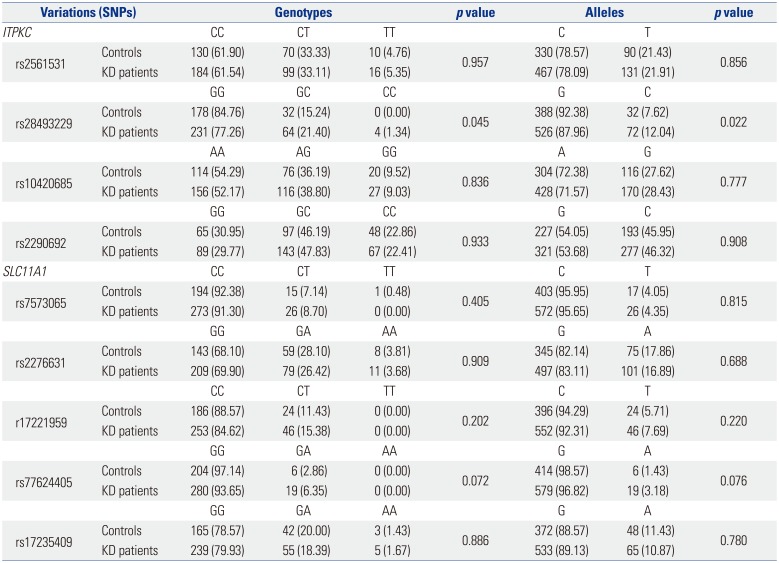
23 In the present study, KD was carefully classified and an appropriate control group was selected to ensure the validity of the results. Patients with atypical or incomplete KD were thus excluded, as were those treated with IVIG before the fourth day of fever. In the present study, a significant increase in the frequency of allele C in rs28493229 was found in KD patients overall. The same increase was observed in KD patients younger than 24 months. Contrary to previous reports from Korea, this result demonstrated that polymorphisms in
ITPKC are indeed associated with KD in Korea. However, the frequency was low (0.12), compared with that reported for other countries, including Japan (0.16).
7
rs28493229 polymorphism in
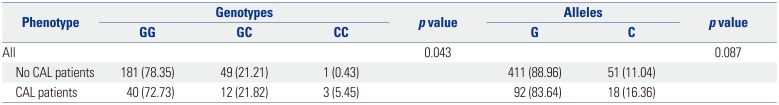
ITPKC was also significantly associated with CALs. In our study, only genotype showed a statistically significant difference but not in alleles. Onouchi
7 reported a significant relationship between the C allele and CALs. In the present study, however, the frequency of the C allele was higher in CALs, although no statistically significant difference was found. In contrast, the same SNP in Taiwanese children was not significantly associated with CALs. These results provide some explanation for the variety of country-specific SNPs.
824
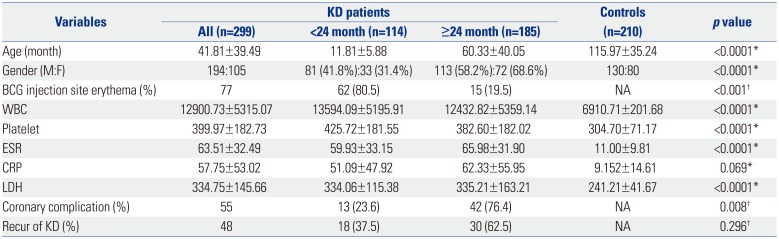
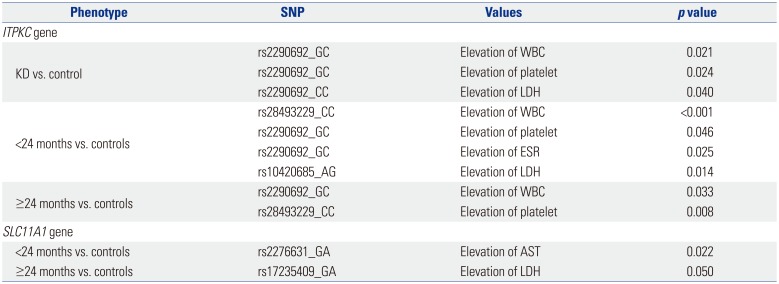
A number of inflammatory markers, such as ESR, CRP, WBC, platelets, and LDH, are typically elevated in the peripheral blood of KD patients during the acute phase of the disease. The patients in the present study also exhibited increased WBC and platelet counts, as well as elevated LDH, CRP levels, and ESR levels. A previous study reported no significant correlations between SNPs in
ITPKC and various inflammatory markers.
8 In the present study, however, several polymorphisms in
ITPKC and
SLC11A1 were associated with elevations in WBC and platelet counts, as well as LDH levels, in the KD group relative to the controls, as shown in
Table 5. The association with elevated inflammatory markers was more pronounced for polymorphisms in
SLC11A1, compared with those in
ITPKC. Furthermore, this association differed in older and younger patients.
The results of this study suggest that KD is not a single disease entity. Differences in age of onset, clinical feature, pattern of laboratory data, and response to IVIG treatment in conjunction with age-related differences in SNPs suggest that KD would be more appropriately designated Kawasaki ‘syndrome’, as it more closely resembles a group of heterogeneous entities that present a similar clinical picture.
A typical laboratory finding in KD is elevated platelet counts. This is very important, because low-dose aspirin treatment is used to prevent thrombosis associated with elevations in platelet count. The exact mechanism underlying the development of thrombocytosis in KD is unknown. Speculatively an increase in serum interleukin (IL)-6 levels during the acute phase of the disease triggers megakaryocyte maturation in the bone marrow, resulting in an increase in the number of platelets in the peripheral blood.
25 According to our data, SNPs in
ITPKC (but not
SLC11A1) are associated with thrombocytosis. These results indicate that
ITPKC is more closely associated with inflammation in KD than
SLC11A1.
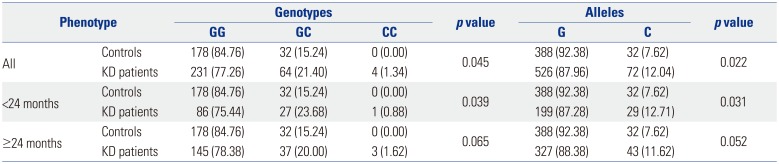
Another interesting finding of the present study is that poly-morphism rs28493229 in
ITPKC has a protective effect against KD symptoms [OR 0.563; 95% confidence interval (CI) 0.343− 0.923;
p=0.022]. We expected that the most-studied
ITPKC SNP rs28493229 would instead have a triggering effect on KD.
26 Some studies have shown no association between KD and polymorphism rs28493229 in
ITPKC.
27 However, the opposite effect was observed in the present study. Along with the previously mentioned increases in many inflammatory cytokines during the acute phase of KD, there is a simultaneous increase in the production of the anti-inflammatory cytokine IL-10.
52628 A number of systems within the human body contribute to the maintenance of homeostasis. When inflammation in the body arises, anti-inflammatory processes work simultaneously to restore homeostasis. Considering this context, the rs28493229 polymorphism in
ITPKC may play a role in suppressing the initial symptoms of KD as a means of maintaining homeostasis.
In the process of choosing tagging SNPs, we added rs2290692 in the 3′-UTR of
ITPKC. Han Chinese KD patients have a higher frequencies of the C allele (
p<0.001), compared with disease-free participants.
22 However, no statistically significant difference in C-allele frequency between KD patients and controls was observed in the present study. This result further demonstrates the geographic differences in the genetic background of KD.
The
SLC11A1 gene was originally named
NRAMP1.
SLC11A1 encodes an iron-transporting protein that plays an important role in controlling susceptibility to
Mycobacterium tuberculosis infection via regulation of IL-1 and TNF production, as well as the activation of macrophages. With respect to BCG, it identifies host genes and proteins that play a key role in the response to mycobacterial infections.
29 As BCG injection site erythema is most common in younger KD patients, we searched for polymorphisms in this gene and found a total of 15 SNPs. Ouchi, et al.
16 showed that one variant of the
SLC11A1 gene is associated with KD in Japan. However, they could not find any polymorphisms in the
SLC11A1 gene, perhaps due to the age of the KD patients enrolled in the study. In contrast to Ouchi, et al.,
16 who did not consider the age of patients in their analysis, in our study, KD patients were dichotomized based on age less than and more than 24 months. In comparing the KD group as a whole with controls, no polymorphisms in
SLC11A1 were found, in agreement with the study of Ouchi, et al.
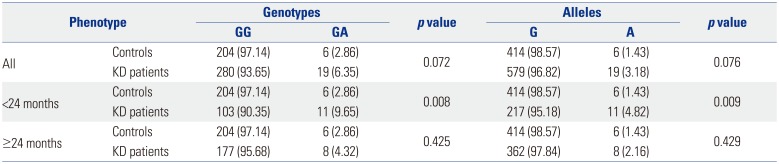
16 However, when KD patients under 24 months of age were considered, a statistically significant polymorphism, rs77624405, was identified, suggesting that this polymorphism in
SLC11A1 is associated with KD in patients under 24 months of age. This finding correlates well with the observed frequency of BCG injection site erythema in patients under 24 months of age. Compared with control subjects, KD patients under 24 months of age tended to have a higher frequency of the A allele in rs2276631, whereas KD patients over 24 months of age tended to have a higher frequency of the A allele in rs17235409.
Gene-gene interactions have been linked to a number of important diseases, such as diabetes and essential hypertension.
3031 In KD, interactions between
ITPKC and the caspase-3 gene have been studied with respect to an association between unresponsiveness of IVIG and development of coronary artery complications.
3233 The potential role of interactions between
ITPKC and
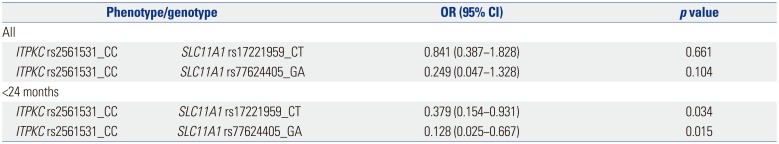
SLC11A1 was therefore examined in the present study. No meaningful interactions between these two genes were observed when the overall KD and control groups were analyzed. However, interactions between rs2561531 in
ITPKC and rs17221959 in
SLC11A1 and between rs2561531 in
ITPKC and rs77624405 in
SLC11A1 were found when the KD patients were considered based on age above and below 24 months, and these interactions were found to have a protective effect. The rs2561531 polymorphism in
ITPKC is particularly interesting. This SNP was not identified in analyses of
ITPKC in the KD patients. In analyses of potential associations between
ITPKC and
SLC11A1, however, a role for rs2561531 in
ITPKC was revealed. Roles for rs17221959 and rs77624405 in
SLC11A1 were also identified.
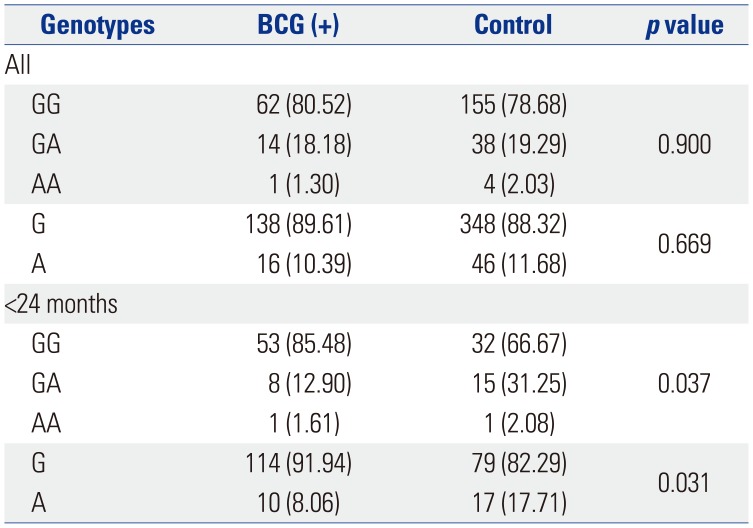
Although BCG injection site erythema is not included in the diagnostic criteria for KD, it is an important clue, as there is no other disease associated with erythematous changes at the BCG injection site. In a previous report, Lin, et al.
8 concluded that there is an association between the C allele of
ITPKC SNP rs28493229 and BCG scars, although the frequency is low (8.04%). However, in the present study, the C allele of
ITPKC SNP rs28493229 was not significantly associated with BCG injection site erythema.
In younger KD patients, the
SLC11A1 SNP rs17235409 was associated with BCG injection site erythema. Although one Japanese study reported no polymorphisms in
SLC11A1,
16 the results may have been affected by differences in nationality, year of introduction of BCG vaccination, or BCG coverage rate. A comparison of patients of all ages with and without BCG injection site erythema indicated that
SLC11A1 SNP rs2276631 was associated with a decreased risk of KD (OR 0.161; 95% CI 0.038−0.689;
p=0.014). In patients less than 24 months of age, however, the
SLC11A1 SNP rs17235409 was associated with increased risk of KD (OR 2.483; 95% CI 1.063−5.804;
p=0.036).
The
SLC11A1 gene is known to be associated with autoimmune and infectious diseases. The SNPs analyzed in this study are associated more with infectious than autoimmune disease. The SNPs rs7573065, rs2276631, rs17221959, and rs17235409 in
SLC11A1 are associated with tuberculosis and infection with
Mycobacterium spp., with one meta-analysis reporting estimated OR >1.
34 As BCG immunization is given to children at less than 1 month of age, the association of these SNPs with KD may be more significant in younger children.
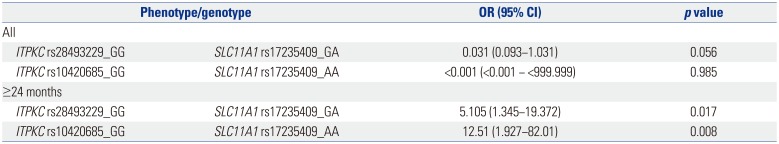
Analyses of interactions between ITPKC and SLC11A1 showed a synergistic effect for BCG injection site erythema in patients over 24 months of age. Interactions between rs28493229 in IT-PKC and rs17235409 in SLC11A1 and between rs10420685 in ITPKC and rs7235409 in SLC11A1 may trigger erythema in BCG injection sites. These results indicate that reactivation of BCG scars is associated with polymorphisms in either ITPKC or SLC11A1 in patients under 24 months of age. However, in patients older than 24 months, reactivation of BCG scars is associated with synergism between the polymorphisms of these two genes rather than either of the genes alone.
During the acute phase of KD, striking immunological derangements occur, including changes in immune cells and marked inflammatory cytokine cascade stimulation.
3 In light of these changes, Kim
3 suggested that acquired immune responses play an important role in the development of KD. Recently, Ikeda, et al.
35 suggested that innate immunity, rather than acquired immunity, plays an important role in the development of vasculitis in KD. Kusuda, et al.
36 reported that KD-specific molecules in the serum possess structures common to microbe-associated molecular pattern (MAMP) molecules of bacteria, such as
Bacillus,
Yersinia, and
Staphylococcus, and that levels of these molecules decline after IVIG treatment. Hara
37 suggested that increased levels of MAMP and damage-associated molecular pattern (DAMP) molecules in the serum of acute-phase KD patients activate the immune system and vascular cells through innate immune pattern recognition receptors, leading to the release of various cytokines. Heat shock protein, which is contained in considerable amounts in the BCG vaccine, is a well-known DAMP. It can thus be speculated that BCG-associated DAMPs play an important role in the development of KD.
Many researchers have concluded that the innate immune system is present at birth and does not change throughout one's life.
38 However, the results of the present study indicate that KD is associated with a difference in genetic background with respect to various inflammatory markers and reactivation of BCG scars in patients younger and older than 24 months. Interestingly, increasing evidence indicates that there are age-associated differences in the maturation and function of the innate immune system.
3940 In light of these factors, the results of the present study show that age-associated differences in BCG injection site erythema and genetic background in patients with KD are reasonable.
It is unclear whether BCG itself induces KD or the BCG injection site is reactivated as a result of specific processes that occur during initiation of KD or possibly whether reactivation of the BCG injection site is caused by the suppression of the inflammation process in KD. Nevertheless, it is clear that the BCG injection site exhibits inflammatory changes that manifest as erythema. Further study is needed to clarify the relationship between BCG injection site erythema and KD, as demonstrated by Chun, et al.
15
This study has several limitations. First, the study was not conducted at the functional gene level. Second, the power of the study was low. Third, the control group was not age-matched. However, it was concluded that age matching would not significantly impact the results, as future development of KD in young, healthy children cannot be ruled out. Therefore, older patients without a history of KD were considered a better choice for the control group.
In conclusion, this study identified polymorphisms in the ITPKC and SLC11A1 genes in Korean KD patients. These polymorphisms were associated with increased production of inflammatory markers in the peripheral blood of patients with KD. The SNPs identified in this study were also associated with erythema of the BCG injection site in KD patients, although their effects differed depending on age of patients.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download