INTRODUCTION
Kawasaki disease (KD) is an acute febrile, multisystemic vasculitis syndrome, occurring mainly in children younger than 5 years of age. It leads to inflammation that affects the small or medium-sized arteries, and this mechanism has clinical manifestations.
1 The most serious complications that can occur in KD are aneurysms or ectasia of the coronary artery and myocardial infarction that can develop due to invasion of the coronary artery.
2
The standard treatment in the acute phase of KD includes high-dose intravenous immunoglobulin (IVIG) and aspirin.
3 The mechanism of IVIG action is still under investigation; however, IVIG appears to have a generalized anti-inflammatory effect involving the inhibition of the interaction between cytokines and endothelial cells. IVIG administration is known to reduce the incidence of coronary artery disease from 20–25% to 2–4%.
4 However, 10–15% of patients with KD show persistent fever despite the administration of initial standard treatments. These patients are considered to be resistant to high-dose IVIG therapy, which in turn increases the risk of coronary artery lesions (CALs) and necessitates more aggressive treatment.
5
The standard treatment protocol for IVIG-resistant KD has not yet been decided. Corticosteroids are the second most commonly used medications to treat patients with KD who have a recurrent or persistent fever after IVIG treatment.
6 Additional second-line drugs for IVIG-resistant KD including methylprednisolone, infliximab, cyclosporine, cyclophosphamide, and methotrexate (MTX) have been reported. However, no standard medication has been established.
3
In 2002, we first reported a patient who was successfully treated for IVIG-resistant KD with MTX.
7 In 2005, MTX was found to be efficacious in four additional patients who were treated for IVIG-resistant KD.
8 In 2008, we reported the use of MTX in 17 patients with a 5-year history of IVIG-resistant KD.
9 Previous studies have shown that MTX is effective in treating IVIG-resistant KD, has no adverse effects, and has economic advantages. By analyzing the data for 10 years, we evaluated the effectiveness of IVIG-resistant KD as a secondary drug and consolidating its position.
MTX is the second most commonly used drug for rheumatoid arthritis. As an inhibitor of dihydrofolate reductase, it is thought to play an anti-inflammatory role in the inflammatory responses of blood vessels in IVIG-resistant KD.
9 In this study, we analyzed the clinical results of the administration of MTX as a therapeutic agent in patients with fever relapse or persistent fever for 10 years.
Go to :

MATERIALS AND METHODS
Study subjects
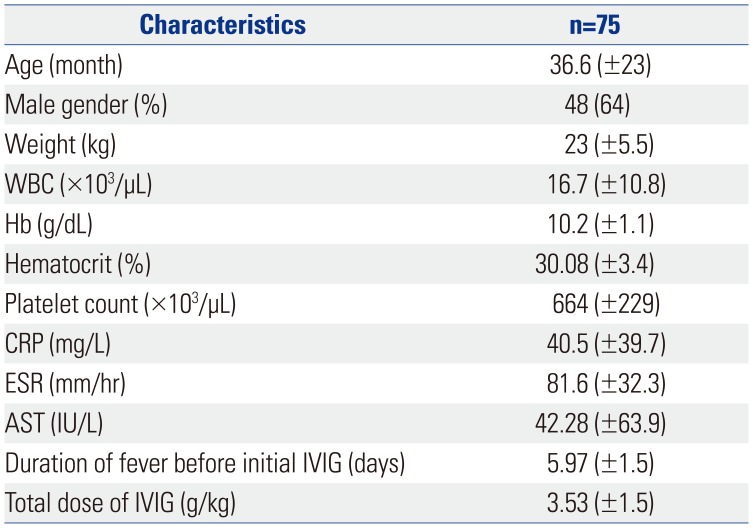
We retrospectively studied the electronic medical records of 80 children admitted to Severance Children's Hospital from July 2005 to November 2015. All the patients had fever for at least 5 days and fulfilled five of the six American Heart Association (AHA) criteria for diagnosing KD (bilateral conjunctivitis; nonexudative mucosal change; erythema, peeling of the lips and strawberry tongue; cervical lymphadenopathy; single node >1.5 cm in diameter or several smaller, firm, polymorphous rashes, commonly maculopapular, which may involve early desquamation in the perineal region as well; erythema and induration of the hands or feet or both). All patients were initially administered high-dose IVIG (2 g/kg) and aspirin (100 mg/kg) within the first 10 days of fever onset. The patients were considered IVIG-resistant when the fever (37.5℃, axillary temperature) persisted or recurred 48 h after treatment with high-dose IVIG and aspirin. Among the 80 patients, five were excluded because their final diagnoses were systemic juvenile rheumatic arthritis, Kikuchi disease, hemophagocytic lymphohistiocytosis, gram-negative sepsis, and reactive arthritis. Finally, 75 cases were included in this study.
This study was approved by the Institutional Review Board of Severance Hospital, Seoul, Korea.
MTX treatment
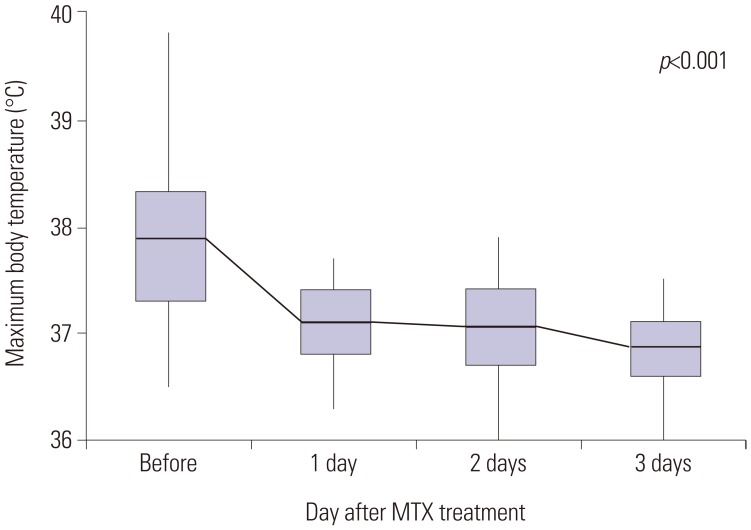
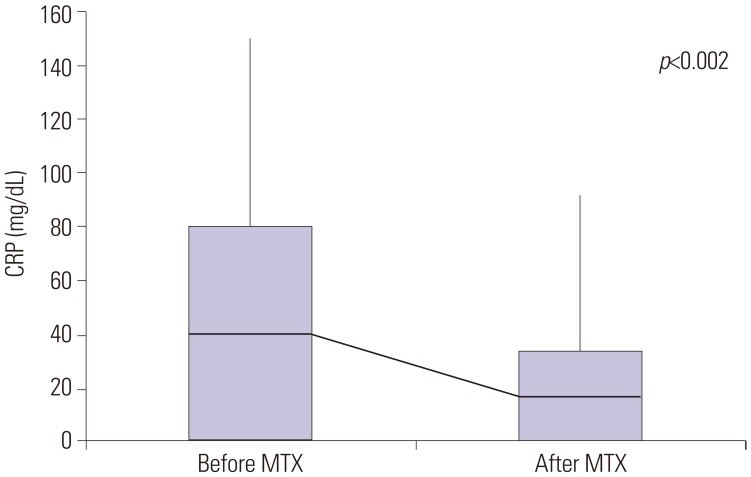
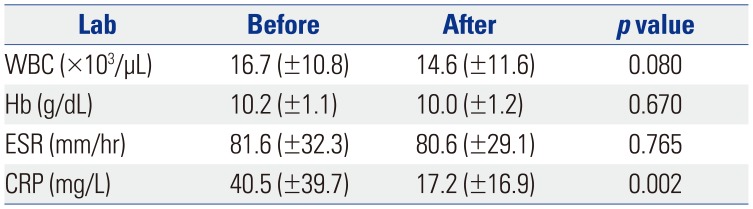
Patients with persistent fever despite an initial treatment with IVIG received repeat IVIG and IV dexamethasone (0.3 mg/kg for 2–3 days) at the same dose. Nonetheless, 75 patients continued to have fever. We repeated the administration of IVIG, steroids, and additional MTX [10 mg/body surface area (BSA)] orally once a week, which is equivalent to the therapeutic dose for juvenile rheumatoid arthritis. The administration of MTX was continued in all patients until the fever subsided and clinical symptoms were relieved. The steroid used at that time was discontinued for 2 to 3 days without tapering out. Day 1 was defined as the first day of MTX administration, and laboratory tests were performed in all patients before and 1 week after treatment. The response to treatment was determined to be non-recurrence of the initial clinical symptoms within 7 days and resolution of fever (axillary temperature <37.5℃). The laboratory tests conducted were determination of white blood cell count, hemoglobin, hematocrit, platelet count, aspartate aminotransferase, albumin, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Echocardiography was performed for each patient on admission and within 1 week of hospitalization and after the fever was absent.
Statistical analysis
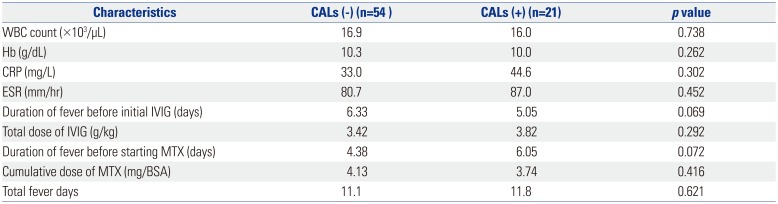
The statistical analyses were conducted using the statistical package for the social sciences (SPSS) software, version 20.0 (IBM Corp., Armonk, NY, USA). Statistical analysis was performed using Wilcoxon matched pairs signed rank test. The categorical data were assessed using the chi-squared test. All p-values <0.05 were considered statistically significant. An independent sample t-test was used to compare the results of two groups with and without CALs.
Go to :

DISCUSSION
Although the cause of KD is still unclear, it is speculated to be a hypersensitivity or abnormal immunological reaction in children with a genetic predisposition induced by offending agents, which may be an infection or inflammation.
10 These processes occur in small- to medium-sized arteries, and generalized vasculitis occurs, invading the entire body and manifesting various clinical symptoms.
11 Approximately 7–9 days after the onset of fever, an influx of neutrophils occurs in the endothelial cells, vascular media, and elastic lamina, followed by the proliferation of CD8+ (cytotoxic) lymphocytes and immunoglobulin A-producing plasma cells.
Mast cells secrete various cytokines [tumor necrosis factor (TNF), vascular endothelial growth factor (VEGF), and monocyte chemotactic and activating factor], interleukins (IL-1, IL-4, and IL-6), and matrix metalloproteinases (MMP3 and MMP9) that target the endothelial cells and cause vascular damage.
1213 In severely affected vessels, the media develops inflammation with necrosis of smooth muscle cells, and the internal and external elastic laminae can split, leading to aneurysms. Over the next few weeks to months, the active inflammatory cells are replaced by fibroblasts and monocytes, and fibrous connective tissue begins to form within the vessel wall.
14 The intima proliferates and thickens while the vessel wall eventually narrows or occludes, owing to stenosis or a thrombus. Recent studies have shown that features, including KD susceptibility, severity, immunopathogenesis, reactivity to IVIG treatment, and coronary artery complications, are associated with genetic susceptibility. It has been shown that innate immunity plays a more important role in immunopathogenesis of disease than acquired immunity, but there are still many areas to be studied.
15
Initial treatment with IVIG (2 g/kg) and aspirin (100 mg·kg
-1·day
-1) reduces the incidence of coronary lesions in KD from 20% to 4%, leading to the rapid onset of the normalization of systemic inflammatory markers.
16 IVIG is known to have a generalized anti-inflammatory effect, and its possible mechanism of action includes the regulation of cytokine production and neutralization of bacterial superantigen.
17
Even with standard treatment with IVIG and aspirin, approximately 10–15% of patients with KD show persistent or recurrent fever (IVIG-resistant KD), which increases their risk for CAL.
18 Several co-treatments have been reported to be used with repeated infusions of IVIG, including IV methylprednisolone, infliximab, abciximab, ulinastatin, MTX, cyclosporine, cyclophosphamide, and plasma exchange.
1519 IV methylprednisolone (30 mg/kg infused for 2 hours and 3 days) rapidly suppresses cytokine levels and subsequently decreases capillary permeability, with outcomes similar to those of patients treated with a second dose of IVIG.
2021 Infliximab (5 mg/kg, IV infusion) binds to TNF-α and prevents its activation. Thereby, IL and proinflammatory cytokines are reduced, and the activity of neutrophils and eosinophils is inhibited.
22 Abciximab, a platelet glycoprotein IIb/IIIa receptor inhibitor, is associated with the resolution of thrombi and vascular remodeling in adults with acute coronary syndromes.
23 Cyclosporine A (CyA) inhibits the assembly and release of IL-2, a cytokine signaling molecule, and inhibits the activation of T lymphocytes.
24 Cyclosporine has not been associated with CAL, but has shown side effects, such as hyperkalemia, hypertension, hirsutism, tremor, and renal dysfunction.
25
In 2002, we reported the successful treatment of a patient with IVIG-resistant KD with MTX, originally.
7 A 6-year-old boy diagnosed with KD was administered IVIG four times; however, the fever persisted even with co-treatment with dexamethasone. On day 31 after the disease onset, MTX (10 mg/BSA, PO once weekly) was initiated and successfully resolved the clinical symptoms. However, a massive bilateral coronary aneurysmal ectasia was confirmed 2 months later by echocardiography, suggesting that MTX did not affect the progression of CALs. In 2005, MTX was reported to be effective in an additional four cases of IVIG-resistant KD in patients with an age distribution ranging from 8 months to 8 years and 9 months.
8 IVIG was administered 2–3 times, and dexamethasone was used for several days over 3–12 days; however, the fever persisted, and low-dose MTX was administered (PO). The fever was controlled in all four patients within 3 days, although there was no improvement of CAL with MTX in this study. In 2008, we reported the use of MTX in 17 patients with IVIG-resistant KD over 5 years. The age of the patients at the disease onset ranged from 4 months to 8 years (median, 2.7 years). All the patients were controlled successfully with MTX, which showed no adverse effects in this study. Furthermore, the number and size of CALs did not decrease, as shown by the echocardiography procedure before and after MTX administration.
In this study, MTX (10 mg/m
2 weekly PO) was used as a secondary agent for KD at the same low doses used in rheumatoid arthritis and appeared to interfere with the hypersensitivity or abnormal immunological reaction.
26 We have not elucidated the exact mechanism by which MTX shows efficacy in IVIG-resistant KD; however, the following mechanisms have been suggested. These include the inhibition of enzymes involved in purine metabolism, leading to the accumulation of adenosine, inhibition of T-cell activation and suppression of intercellular adhesion molecules by T-cells, and selective downregulation of B-cells.
2728 Another proposed mechanism of action of MTX is the inhibition of methyltransferase activity, causing the inactivation of enzyme activity related to immune system function. These mechanisms may have contributed to the rapid fever resolution and improvement of inflammatory markers experienced by these patients treated with MTX. These results suggest that MTX is a potential candidate drug for second-line therapy of IVIG-resistant KD. The administration of MTX to children is challenging because of its various side effects. In addition, MTX is used as an anticancer drug and, thus, it may create a sense of discomfort for patients and caregivers. Nevertheless, none of the patients enrolled in this study showed the common side effects of MTX, such as leukopenia, hepatotoxicity, and ulcerative stomatitis.
29
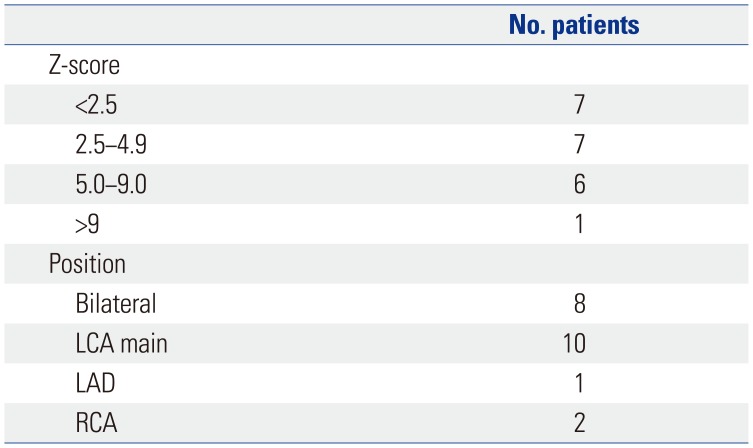
The most serious complication of KD is CAL, and we have considered this from two perspectives. First, in patients with CAL, MTX was used to confirm the improvement, but no statistically positive results were obtained. Second, we observed that MTX could not prevent the development, formation, or aggravation of the observed CAL, which was not resolved. Our study had a few limitations including the concomitant use of steroids, which makes it difficult to assess the effect of MTX alone. In the case of recurrence of fever after initial IVIG treatment, MTX was administered to IVIG and steroid for an average of 3.2 days, and the steroid used at that time was discontinued without tapering out. The steroids administered with MTX were discontinued after a single dose, and because MTX was administered weekly to treat fever and symptoms, we determined that MTX played an important role in IVIG-resistant KD. The use of steroids did not appear to improve symptoms because MTX was added when the fever subsided. In addition to the clinical improvement described above, MTX has been administered for 10 years, confirming that no particular side effect is associated with its use and, thus, it is superior as a second-line drug. In addition to steroids, we have used cyclosporine in patients with IVIG-resistant KD, although we did not continue to do so because of poor results in the control of fever or improvement in the test. Second, this was not a controlled study. We retrospectively analyzed the data of patients administered MTX obtained from an electronic medical record database. Therefore, there was no control in the design of subject recruitment and study. Third, this study was conducted in a single center and, therefore, MTX therapy should further be assessed in a multicenter, placebo-controlled trial.
In conclusion, MTX effectively treated IVIG-resistant KD, improved the patients' clinical symptoms, and reduced the acute phase reactants. However, MTX did not affect the improvement or prevention of CAL. Finally, MTX could be a candidate for standard treatment for IVIG-resistant KD.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download