Abstract
Purpose
To determine the efficacy of cognitive targeted prostate biopsy using biparametric magnetic resonance imaging (b-MRI) for patients with prostate-specific antigen levels under 10 ng/mL.
Materials and Methods
We reviewed data from 123 consecutive patients who underwent cognitive targeted prostate biopsy using prostate MRI. Of these patients, the first 55 underwent prostate biopsy using multiparametric MRI (mp-MRI), and the remaining 68 underwent prostate biopsy using b-MRI. For b-MRI, we generated T2 weighted axial imaging and diffusion-weighted imaging sequences. We found that 62 of the 123 men had suspicious lesions on MRI (32 of the 55 men in the mp-MRI group and 30 of the 68 men in the b-MRI group). We compared the prostate cancer detection rates and the proportions of clinically significant prostate cancer between the different MRI sequences.
Results
Between the two MRI groups, there were no statistically significant differences in prostate cancer detection rate and proportions of clinically significant prostate cancer (41.8% vs. 30.9%, p=0.208 and 82.6% vs. 76.2%, p=0.598). Among the 62 men who had suspicious lesions on MRI, the prostate cancer detection rates were 62.5% and 63.3% (p=0.709) in the mp-MRI and b-MRI groups, respectively, and the proportions of clinically significant prostate cancer were 95.0% and 84.2% (p=0.267).
Conclusion
Prostate biopsy using b-MRI showed similar performance to that using mp-MRI for detecting prostate cancer and clinically significant prostate cancer. Considering the satisfactory performance and cost effectiveness of b-MRI, this technique could be a good option for obtaining intraprostatic information for first round prostate biopsy.
Transrectal ultrasound-guided prostate biopsy (TRUS-Bx) is the gold standard for detecting prostate cancer in patients with elevated prostate-specific antigen (PSA) levels. However, TRUS-Bx often results in misdiagnosis or misclassification of patients who have clinically significant prostate cancer.1234 For example, some patients appear to have no disease even though they have prostate cancer, and others are classified as active surveillance (AS) candidates even though they need curative therapy. Due to its potential ability to overcome these limitations, prostate biopsy using multiparametric magnetic resonance imaging (mp-MRI) has received attention recently.
According to several recent studies, targeted prostate biopsy using mp-MRI is efficacious in clinically significant prostate cancer detection.5678 Nevertheless, this biopsy technique is not popular because of its high cost. The costs of TRUS/MRI fusion Bx and bore MRI-Bx are even greater than that of the cognitive targeted prostate biopsy technique, because the former techniques require more specialized equipment. For these reasons, targeted prostate biopsy using mp-MRI is often performed as a repeat biopsy technique for men with persistently elevated PSA, despite a negative result of first round prostate biopsy.
Generally, the cost and time required for MRI are associated with the number of image sequences. If fewer MRI sequences are used, the cost of MRI is reduced, and the stay time in-bore is also decreased. We, therefore, decided to perform cognitive targeted prostate biopsy using biparametric MRI (b-MRI) for first prostate biopsy of patients with a PSA level under 10.0 ng/mL. Herein, we report the results of cognitive targeted prostate biopsy using b-MRI in comparison to those of cognitive targeted prostate biopsy using mp-MRI. We also report and compare the proportions of clinically significant prostate cancer cores.
In 2016, a total of 464 patients underwent prostate biopsy at Pusan National University Yangsan Hospital (Yangsan, Korea). Of these 464 patients, 338 underwent prostate biopsy due to PSA level under 10.0 ng/mL. Before prostate biopsy, each urologist explained the MRI-Bx technique to the patient; the final choice regarding the use of the Bx technique was left to each patient. A total of 207 patients chose TRUS-Bx, and 131 patients chose the MRI-Bx technique. Of the 131 patients who underwent MRI-Bx, we excluded eight who were undergoing a repeat prostate biopsy. Finally, we reviewed the pathologic results of 123 patients in the MRI-Bx group. Among these 123 patients, the first 55 underwent mp-MRI including T2-weighted images (T2WI) in three orthogonal planes (axial, sagittal, and coronal), dynamic contrast-enhanced imaging (DCI), and diffusion-weighted imaging (DWI). The remaining 68 patients underwent b-MRI, including axial T2WI and DWI.
In our institute, all patients undergo 3.0 T MRI (InteraAchieva 3.0 T, Phillips Medical System, Best, the Netherlands) on an instrument equipped with a phased-array coil (six-channel). The routine 3.0 T prostate mp-MRI protocol consists of T2WI in three orthogonal planes (axial, sagittal, and coronal), DCI, and DWI. The MRI procedure costs approximately 600 US dollars. The average scanning time for T2WI with three orthogonal planes is approximately 12 minutes. The average DCI time is about 20 minutes, and the average DWI time is about 8 minutes. Thus, routine mp-MRI requires a total of 45 minutes, including time for patient preparation.
For b-MRI, axial T2WI for anatomical evaluation and DWI scans were acquired within 4 minutes and 8 minutes, respectively. Two b-values (0–1000) were used, and diffusion restriction was quantified through apparent diffusion coefficient (ADC) mapping by the scanner program. The total scan time was about 15 minutes, including patient preparation. We set the cost of this b-MRI at 300 US dollars considering the number of MRI sequences involved.
All prostate MRI imaging scans were interpreted by two experienced uroradiologists. The uroradiologists denoted suspicious regions of interest on ADC maps on a Digital Imaging and Communications in Medicine (DICOM) workstation using the information from the present mp-MRI/b-MRI sequences. A modified three-grade scoring system was used (based on the PI-RADS scoring system version 2.0) as follows: 1, weakly suspicious lesion (probably benign); 2, moderately suspicious lesion; or 3, highly suspicious lesion.
One urologist (D.H. Lee) performed all MRI-Bx procedures for the entire study period. All patients were prepared with local gel anesthetics using a BK ultrasound scanner, an endfire transducer, a needle guide, and an 18-G 25-cm biopsy needle. The operator reviewed the MRI images. Any suspicious lesions on MRI were visually matched and registered on the corresponding axial TRUS image based on zonal anatomy. All MRI-Bx cores were marked and kept in separate bottles according to the location of the suspicious lesion on the MRI image. After MRI-Bx, TRUS-Bx cores were collected from 12 prostatic regions and marked separately. Patients without suspicious lesions on MRI underwent TRUS-Bx only.
A genitourinary pathologist reviewed and described all biopsy cores. For each positive biopsy core for prostate cancer, Gleason score and cancer core length were reported. To evaluate the clinical significance of prostate cancer, we defined a clinically significant prostate cancer core as having a cancer core length greater than 5 mm and/or a Gleason grade greater than 3. Additionally, we evaluated 24 radical prostatectomy (RP) specimens of patients who underwent RP after MRI-Bx in our institute and 26 RP specimens of patients with prostate cancer who were diagnosed by TRUS-Bx. We obtained the postoperative Gleason score, pathologic stage, and tumor volume.
We compared the pathologic results of mp-MRI versus b-MRI biopsy cores using chi-square tests and independent t-tests for categorical and continuous variables, respectively. We also compared the prostate cancer detection rates and proportions of clinically significant prostate cancers between the two groups. Also, using RP specimens from an additional 50 patients we evaluated, we preoperatively identified AS candidates and evaluated whether each candidate had insignificant prostate cancer. In the present study, we defined AS candidates as patients with organ-confined prostate cancer with GS6 for less than two cores and a core cancer length shorter than 5 mm. For RP specimens, insignificant prostate cancer was defined as organ-confined, Gleason 6 disease with tumor volume <0.5 cm3. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). p values ≤0.05 were considered statistically significant.
The clinicopathologic characteristics of the patients are shown in Table 1. The cost was approximately 600 US dollars for mp-MRI and 300 US dollars for b-MRI. The stay time in-bore was about 45 minutes for mp-MRI and 15 minutes for b-MRI. The overall prostate cancer detection rate was 23 of 55 (41.8%) in the mp-MRI group and 21 of 68 (30.9%) in the b-MRI group (p=0.208). Although the overall prostate cancer rate was lower in the b-MRI group, this difference was not statistically significant. The proportions of clinically significant cancer were also not statistically significantly different between the two groups (19 of 23, 82.6% vs. 16 of 21, 76.2%; p=0.598).
We also separately analyzed the results of patients who had suspicious lesions on MRI scans. Suspicious lesions were observed more often in the mp-MRI group than in the b-MRI group, although this difference was not statistically significant (32 of 55, 58.2% vs. 30 of 68, 44.1%; p=0.121). The overall prostate cancer detection rates and proportions of clinically significant cancers were not statistically significantly different between the two groups. In the 62 patients who had suspicious lesions on MRI, the overall prostate cancer detection rate was 20 of 32 (62.5%) in the mp-MRI group and 19 of 30 (63.3%) in the b-MRI group (p=0.946). The proportions of clinically significant cancers were 19 of 20 (95.0%) in the mp-MRI group and 16 of 19 (84.2%) in the b-MRI group (p=0.267). Also, we compared the diagnostic accuracy using the sensitivity and specificity between mp-MRI and b-MRI. There were no statistical differences between the two (Table 2).
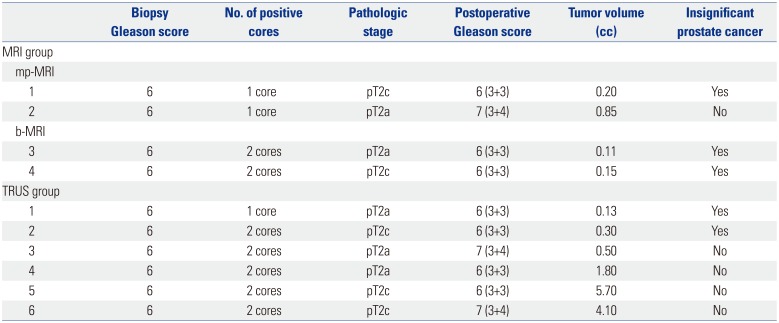
We also reviewed 50 prostate specimens from the patients who underwent RP in our institute during the study period (Table 3). Of these specimens, we classified four of the 24 (16.6%) AS candidates in the MRI-Bx group and six of the 26 (23.0%) in the TRUS-Bx group. We evaluated the RP specimens of the AS candidates and found that three of the four AS candidates in the MRI-Bx group actually had insignificant prostate cancer that was suitable for AS. However, among the 6 patients in the TRUS-Bx group, only two were AS candidates. The other patients had prostate cancer that required curative treatment (Table 4).
Generally, mp-MRI adequately demonstrates prostate anatomy and provides intraprostatic information using the imaging function of MRI. This technique offers information regarding the precise tumor localization, tumor size, and accurate prostate cancer staging.9101112 Accurate tumor location and size information enables doctors to obtain qualified prostate samples during targeted prostate biopsy. Nevertheless, MRI-Bx is not popular because of its high cost and the additional time required in an MRI machine. In particular, to obtain DCI, gadolinium-based contrast agents must be administered via i.v. access. These agents are relatively expensive and can potentially accu-mulate in deep cerebral structures.13 Moreover, if patients request to undergo in-bore MRI biopsy or MRI/TRUS fusion biopsy, the cost is even greater because these MRI-Bx techniques require additional specific devices and programs. Therefore, cost-related barriers preclude the use of MRI-Bx in first-round Bx, even though this technique has several advantages. Thus, cost reduction of MRI is important: decreasing the number of MRI sequences is a promising approach to achieving cost reduction.
In this context, several groups recently reported the utility of using only T2WI and DWI images for detecting prostate cancer. Rais-Bahrami, et al.14 showed that, when b-MRI was combined with PSA level and PSA density, even higher sensitivity and specificity were achieved, providing greater diagnostic accuracy in detecting clinically significant disease. These data provide support for limited non-contrast MRI as a potential adjunct tool to optimize prostate cancer detection. Also, Fascelli, et al.15 validated the use of the b-MRI protocol along with the use of PSA or PSA density in a biopsy-naive cohort of 59 patients at risk for prostate cancer. The authors concluded that the combined use of b-MRI, PSA, and PSA density results in improved diagnostic accuracy for detecting clinically significant prostatic carcinoma.
In the present study, we also used b-MRI for cognitive targeted prostate Bx. In the early phase of the study, we used mp-MRI, including all MRI sequences (i.e., T2WI, DWI, and DCI). The MRI cost was approximately 600 US dollars, and about 45 minutes of imaging time was required to obtain all necessary images. Using these MRI sequences, we showed that our approach had significant efficacy to detect clinically significant prostate cancer.8 Although we showed the efficacy of MRI in first round prostate biopsy, the cost and extended time in-bore hindered the application of this biopsy technique. Therefore, we changed the MRI protocol in our institute for patients with economic concerns. Our revised protocol consisted of only two sequences: axial T2WI for anatomical evaluation and DWI. These sequences were acquired within 4 minutes and 8 minutes, respectively. The cost for the MRI procedure was reduced to 300 US dollars from 600 US dollars. As we discussed in the Results, the overall detection rates of prostate cancer and clinically significant prostate cancer were not different between the mp-MRI and b-MRI groups. Also, the diagnostic accuracy was not statistically different. We thought that this results was caused by the characteristic of DCI. In DCI, it is beneficial for the prostate cancer patients who had larger tumor.1617 In the present study, the study cohort was limited as patients who had PSA levels under 10 ng/mL. In patients who had low PSA, they tended to have smaller tumor volume. Thus, DCI did not affect the diagnostic accuracy. Accordingly, without T2WI (sagittal and coronal) and DCI, the targeted prostate biopsy results were not inferior to those of full mp-MRI sequences.
If DCI was performed for MRI-Bx, it would be a helpful technique for detecting prostate cancer. However, Cheikh, et al.16 reported that DCI did not have any significant differences, compared with T2WI. In their study, DCI was significantly less specific (83.5% vs. 89.7%, p<0.002) than T2WI. Moreover, while DCI was more sensitive (52.4% vs. 32.1%), the difference was not significant (p=0.09). Delongchamps, et al.17 also reported that DCI did not increase the accuracy, compared with DWI, for the detection of prostate cancer in either the prostate peripheral zone (PZ) or transitional zone (TZ). Moreover, they concluded that DCI significantly decreased the accuracy. Furthermore, according to the PI-RADS v2 guidelines published in December 2014 for evaluating PZ lesions, DWI is the dominant sequence, while the T2WI sequence plays a primary role for evaluating TZ lesions.18 Consequently, DCI sequences have been assigned a secondary role, because they are only useful for providing additional information for PZ lesions with a score of 3. Therefore, DCI plays a role as the minor sequence when PZ cancer is equivocally suspected at DCI.
When we evaluated RP specimens, b-MRI seemed more capable of predicting insignificant prostate cancer than mp-MRI. Among the patients who underwent RP with clinically significant prostate cancer according to b-MRI targeted prostate biopsy, all had significant prostate cancer. Moreover, all AS candidates according to b-MRI targeted prostate biopsy had clinically insignificant disease. A similar finding was noted in the mp-MRI group. However, TRUS-Bx showed only about 33% accuracy in predicting AS candidates in the present study. Although we did not observe a statistically significant difference due to the small number of AS candidates who underwent RP, we predict that examination of RP specimens would enhance the performance of b-MRI. This is a major limitation of the present study.
Also, the small number of study patients is another limitation of this study. Even though we described the diagnostic accuracy according to specificity and sensitivity to detect prostate cancer in the present study, we agree that the statistical power was not strong due to the small study cohort. However, the aim of the present study was not to characterize the statistical predictive power of the method, but to report a comparison of pathologic results according to MRI technique. We already compared the performance of mp-MRI with that of conventional TRUS-Bx using a similar study cohort of men with a PSA level under 10 ng/mL who underwent first round prostate biopsy. We, therefore, reasoned that a pathologic comparison between the mp-MRI group and the b-MRI group could help demonstrate the efficacy of b-MRI targeted prostate biopsy.
Recently, de Rooij, et al.19 reported that the MRI strategy is cost-effective, compared with the standard of care using TRUS-Bx, even though the presented cost-effectiveness estimates had some uncertainty. They concluded that the total costs of the MRI strategy are almost equal with the standard of care, while the potential reduction of overdiagnosis and overtreatment with the MRI strategy leads to an improvement in the QoL of patients with prostate cancer. According to this study, the MRI strategy could replace conventional TRUS imaging or additional imaging approaches that should be performed before TRUS-Bx. Since b-MRI has a lower cost, requires less time, and does not require the administration of contrast by i.v. access, we can gain similar advantages with mp-MRI. We, therefore, discerned that b-MRI is potentially useful for detecting prostate cancer in all patients who require an immediate first round prostate biopsy.
In conclusion, targeted prostate biopsy using b-MRI showed similar performance to that using mp-MRI for prostate cancer detection and clinically significant prostate cancer detection in patients with a PSA level under 10 ng/mL. Considering the good performance and cost-effectiveness of b-MRI, this technique is a good option for obtaining intraprostatic information for first round prostate biopsy.
ACKNOWLEDGEMENTS
This study was supported by Research Institute for Convergence of Biomedical Science and Technology (30-2015-027), Pusan National University Yangsan Hospital.
References
1. Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009; 101:374–383. PMID: 19276453.

2. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010; 102:605–613. PMID: 20413742.

3. Lee DH, Koo KC, Lee SH, Rha KH, Choi YD, Hong SJ, et al. Low-risk prostate cancer patients without visible tumor (T1c) on multiparametric MRI could qualify for active surveillance candidate even if they did not meet inclusion criteria of active surveillance protocol. Jpn J Clin Oncol. 2013; 43:553–558. PMID: 23580758.

4. Bul M, Zhu X, Rannikko A, Staerman F, Valdagni R, Pickles T, et al. Radical prostatectomy for low-risk prostate cancer following initial active surveillance: results from a prospective observational study. Eur Urol. 2012; 62:195–200. PMID: 22342775.

5. Selnæs KM, Heerschap A, Jensen LR, Tessem MB, Schweder GJ, Goa PE, et al. Peripheral zone prostate cancer localization by multiparametric magnetic resonance at 3 T: unbiased cancer identification by matching to histopathology. Invest Radiol. 2012; 47:624–633. PMID: 23011187.
6. Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010; 255:89–99. PMID: 20308447.
7. Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013; 64:713–719. PMID: 23787357.

8. Lee DH, Nam JK, Park SW, Lee SS, Han JY, Lee SD, et al. Visually estimated MRI targeted prostate biopsy could improve the detection of significant prostate cancer in Patients with a PSA Level <10 ng/mL. Yonsei Med J. 2016; 57:565–571. PMID: 26996553.
9. Rastinehad AR, Baccala AA Jr, Chung PH, Proano JM, Kruecker J, Xu S, et al. D'Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011; 185:815–820. PMID: 21239006.

10. Yerram NK, Volkin D, Turkbey B, Nix J, Hoang AN, Vourganti S, et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int. 2012; 110(11 Pt B):E783–E788. PMID: 23130821.

11. Stamatakis L, Siddiqui MM, Nix JW, Logan J, Rais-Bahrami S, Walton-Diaz A, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013; 119:3359–3366. PMID: 23821585.

12. Rais-Bahrami S, Siddiqui MM, Turkbey B, Stamatakis L, Logan J, Hoang AN, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013; 190:1721–1727. PMID: 23727310.

13. Stojanov D, Aracki-Trenkic A, Benedeto-Stojanov D. Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents-current status. Neuroradiology. 2016; 58:433–441. PMID: 26873830.

14. Rais-Bahrami S, Siddiqui MM, Vourganti S, Turkbey B, Rastinehad AR, Stamatakis L, et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU Int. 2015; 115:381–388. PMID: 24447678.

15. Fascelli M, Rais-Bahrami S, Sankineni S, Brown AM, George AK, Ho R, et al. Combined biparametric prostate magnetic resonance imaging and prostate-specific antigen in the detection of prostate cancer: a validation study in a biopsy-naive patient population. Urology. 2016; 88:125–134. PMID: 26680244.

16. Cheikh AB, Girouin N, Colombel M, Maréchal JM, Gelet A, Bissery A, et al. Evaluation of T2-weighted and dynamic contrast-enhanced MRI in localizing prostate cancer before repeat biopsy. Eur Radiol. 2009; 19:770–778. PMID: 18925403.

17. Delongchamps NB, Rouanne M, Flam T, Beuvon F, Liberatore M, Zerbib M, et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 2011; 107:1411–1418. PMID: 21044250.

18. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur Urol. 2016; 69:16–40. PMID: 26427566.
19. de Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health car+A1:B20e perspective. Eur Urol. 2014; 66:430–436. PMID: 24377803.
Table 1
Comparison of Pathologic Results of Prostate Biopsy According to MRI Sequence
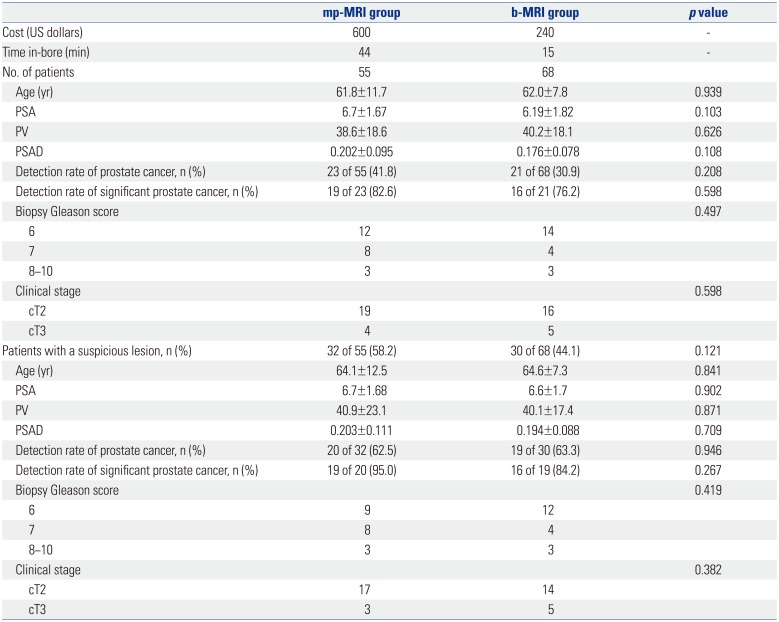
Table 2
Diagnostic Accuracy of Prostate Cancer Detection of a Suspicious Lesion between mp-MRI and b-MRI
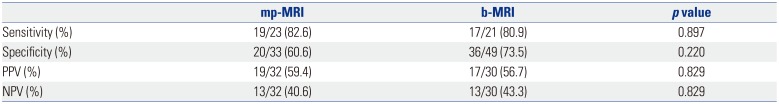
| mp-MRI | b-MRI | p value | |
|---|---|---|---|
| Sensitivity (%) | 19/23 (82.6) | 17/21 (80.9) | 0.897 |
| Specificity (%) | 20/33 (60.6) | 36/49 (73.5) | 0.220 |
| PPV (%) | 19/32 (59.4) | 17/30 (56.7) | 0.829 |
| NPV (%) | 13/32 (40.6) | 13/30 (43.3) | 0.829 |
Table 3
Pathologic Outcomes after RP According to Biopsy Technique
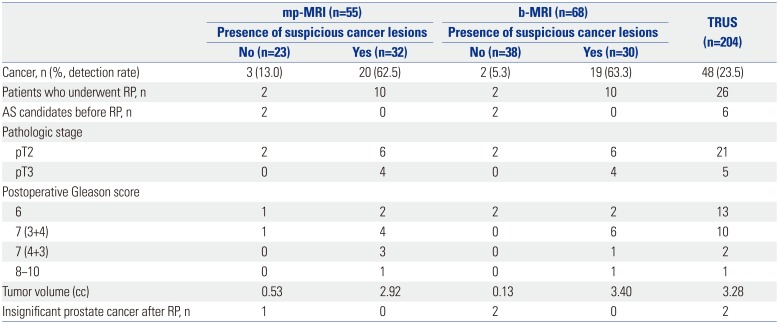
Table 4
Radical Prostatectomy Results of Active Surveillance Candidates after Prostate Biopsy (n=10)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download