Abstract
Purpose
This study compared the impact of paclitaxel-coated balloons (PCB) or drug eluting stents (DES) on peri-procedural myocardial infarction (PMI) on de novo coronary lesion in stable patients.
Materials and Methods
In this observational study, we compared the incidence of PMI amongst patients with single vessel de novo coronary lesions who underwent treatment with a PCB or DES. Propensity score-matching analysis was used to assemble a cohort of patients with similar baseline characteristics. PMI was classified as myocardial infarction occurring within 48 hours after percutaneous coronary intervention with a threshold of 5 x the 99th percentile upper reference limit of normal for creatine kinase-myocardial band (CK-MB) or troponin T (TnT).
Results
One hundred four patients (52 receiving PCB and 52 receiving DES) were enrolled in this study. The peak mean values of CK-MB and TnT were significantly higher in the DES group. There was a significantly higher rate of PMI in the DES group (23.1% vs. 1.9%, p=0.002). Total occlusion of the side-branch occurred in two patients treated with DES, while no patients treated with PCB. In multivariable analysis, DES was the only independent predictor of PMI compared with PCB (odds ratio 42.85, 95% confidence interval: 3.44–533.87, p=0.004).
About one-third of all elective percutaneous coronary intervention (PCI) procedures are associated with significant myocardial injury, termed peri-procedural myocardial infarction (PMI), which is characterized by cardiac biomarker elevation.1 Although the clinical significance of small PMI as characterized by low-level biomarker elevation remains controversial,23 many studies have suggested that PMI is associated with an increased risk of mortality during long-term follow-up.4567 Whereas the use of drug eluting stents (DESs) has dramatically reduced the rate of restenosis,8 up to 30% of cases are still complicated by PMI.910 The most common mechanism of PMI is side-branch occlusion by foreign object such as stent struts through both plaque shift from the main vessel and carina shift.911 In the DES era, most side-branch occlusions occur after post-stent dilation, which is performed with high-pressure balloon inflation.12 In this regard, paclitaxel-coated balloon (PCB) treatment is an attractive therapeutic option and may have benefits over DES. In the absence of stent struts, PCB has considerably less chance of side-branch occlusion in theory, compared to stents. However, the impact of treatment with PCB compared to DES on PMI has not been previously investigated. Therefore, the aim of this study was to compare the impact of PCB versus DES on PMI in stable patients.
This population-based cohort study was conducted using the DES database at Ulsan University Hospital. In total, 679 patients who underwent successful elective PCI with DES were enrolled from March 2004 through August 2007, and patients treated successfully with PCB were enrolled from April 2011 through July 2013. Patients with stable angina pectoris who were scheduled to undergo elective PCI for de novo coronary single lesions were enrolled if they had lesions with a ≥70% diameter stenosis, a reference vessel diameter of more than 2.0 mm and lesion length of ≤24 mm. In the PCB group, all patients were unable to receive long-term dual antiplatelet therapy due to high bleeding risk, poor compliance or because they await for non-cardiac surgery. Of these patients, only those who had documented simultaneous measurements of creatine kinase-myocardial band (CK-MB) and troponin T (TnT) at baseline and 8-hour intervals post-PCI for 24 hours (until the time to decrease from the peak values of cardiac biomarkers within 48 hour after procedure) were included in our analysis. In this study, to reduce the effect of treatment selection bias and other confounding, with different recruitment periods, we performed propensity score-matching. To adjust potential confounders, a propensity score-matched analysis was performed using binomial logistic regression analysis including the following covariates: target vessel of PCI, diagnosis, diameter of device and length of device.
Exclusion criteria were multi-vessel disease, acute myocardial infarction preceding the index procedure, bypass grafting, bifurcation lesions treated with a 2-stent strategy, left ventricular ejection fraction <30%, left main disease, heavily calcified or thrombotic lesions, life expectancy <1 year and known chronic kidney disease (creatinine >2 mg/dL).
The definition of myocardial infarction was a threshold of 5 x the 99th percentile upper reference limit of normal for CK-MB or TnT.13 PMI was classified as myocardial infarction occurring within 48 hours after PCI.14 Death was considered cardiac in origin unless obvious non-cardiac causes were identified. Target vessel revascularization was defined as PCI of the target lesion or any segment of the epicardial coronary artery containing the target lesion. Target lesion revascularization was defined as any clinically driven repeat revascularization caused by a >50% stenosis within the PCI site or within a 5-mm border proximal or distal to the PCI site. Side-branch occlusion was defined as Thrombolysis In Myocardial Infarction flow grade 0 or 1 after device use. Side-branch compromise was defined as ≥70% diameter stenosis of side-branch >1.5 mm arising from the culprit lesion after device apply. The clinical outcomes were cardiac death, myocardial infarction, target vessel revascularization and target lesion revascularization. This study was carried out according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board Ethics Committee. All enrolled patients provided written informed consent.
All patients were treated with acetylsalicylic acid 200 mg and loading dose of clopidogrel 300 mg before the procedure followed by maintenance clopidogrel 75 mg daily for at least 12-months in DES group and for 6 weeks and for extended periods thereafter at the physician's discretion in PCB group. DESs for all implanted lesions were Cypher™ (Cordis, Johnson & Johnson Co., New Jersey, FL, USA), Taxus Express™ (Boston Scientific, Natick, MA, USA) and Endeavor™ (Medtronic Co., Minneapolis, MN, USA). For PCB treatment, the standard balloon was shorter than the intended PCB size, and the PCB (SeQuent Please®, PCB catheter, B. Braun, Melsungen, Germany) was inflated at nominal pressure for 60 seconds. Post-dilation was not performed in all PCB cases. Coronary angiographies before and after the procedure were analyzed using the Cardiovascular Angiography Analysis System (CAAS 5.10, Pie Medical Imaging B.V., Maastricht, the Netherlands) by an independent investigator, who was blinded to clinical presentations.
All statistical analyses were done using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA). Descriptive statistical methods were used to describe the data. Results are presented as mean±standard deviation for continuous variables and frequency (percentages) for categorical variables. After propensity matching, comparisons between the two groups were performed using the paired t-test for continuous variables and the McNemar test for categorical variables. To identify the independent predictors of PMI, we performed multivariable logistic regression analysis using the following covariates: all variables with values of p<0.10 on the univariate analysis and those judged to be of clinical significance. The multivariable model was created with a stepwise elimination procedure, where the independent variables were entered into the model at the 0.20 significance level and removed at the 0.25 level. All tests were two-sided, and a p-value <0.05 was considered statistically significant.
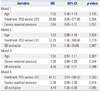
The matched cohort included 104 patients who had been successfully treated with PCB (n=52 patients) and DES (n=52 patients). Baseline clinical characteristics of the patients are shown in Table 1. The prevalence of past history of dyslipidemia was lower in DES group than in PCB group. The peak mean values of CK-MB (6.4±8.5 ng/mL vs. 2.0±1.8 ng/mL, p<0.001) and TnT (0.14±0.20 ng/mL vs. 0.06±0.07 ng/mL, p=0.007) were significantly higher in DES group than PCB group.
The procedural, angiographic characteristics and incidence of PMI are shown in Table 2. The maximal pressure in DES group was higher than in PCB group (13.1±2.8 mm Hg vs. 8.2±1.8 mm Hg, p<0.001). Side-branch occlusion occurred only in 2 patients treated with DES (DES: 3.8% vs. PCB: 0%, p=0.495). Similarly, slow- or no-reflow following device use occurred in only 2 patients treated with DES. In pre-procedure, there were no significant between-group differences in the reference vessel diameter, minimal lumen diameter (MLD), diameter stenosis and lesion length. In post-procedure, the DES group had a significantly higher MLD (2.4±0.4 mm vs. 1.7±0.4 mm, p<0.001) and a smaller diameter stenosis (9±6% vs. 31±12%, p<0.001). The incidence of PMI was significantly higher in the DES group (23.1%, n=12 vs. 1.9%, n=1, p=0.002). At 12-months follow-up, the clinical events observed were cardiac death, myocardial infarction, target vessel revascularization and target lesion revascularization which occurred with no significant differences between the 2 groups (cardiac death: PCB, 0%, n=0 vs. DES, 0%, n=0, p=1.000; target vessel revascularization: PCB, 1.9%, n=1 vs. DES, 1.9%, n=1, p=1.000; target lesion revascularization: PCB, 1.0%, n=1 vs. DES, 0%, n=0, p=1.000).
The covariates entered into the final logistic regression analysis included age, treatment with PCB/DES, maximal pressure of device, lesion length after device use, reference diameter after device use, MLD after device use, diameter stenosis after device use and side-branch occlusion by univariate analysis. The predictors of PMI by binomial logistic regression analysis are shown in Table 3. Because of low incidence of PMI, we used multi-model logistic regression analysis including 3 covariates in each analysis. The use of DES was the only independent predictor of PMI (p<0.05 in all models).
This propensity score-matched analysis evaluated the impact of PCB or DES on PMI in stable patients, and demonstrated that treatment with a PCB was associated with a significant reduction in the risk of PMI.
PMI has been reported to be associated with increased adverse clinical outcomes.6151617 A large meta-analysis showed higher mortality for increasing levels of CK-MB; with even a minor increase of CK-MB 1-3x the upper limit of normal conferring a 1.5 times higher relative risk of death [95% confidence interval (CI): 1.2–1.8].1 A later meta-analysis showed that any troponin elevation was associated with a significantly increased risk of mortality (4.4% vs. 3.3%, p=0.001; odds ratio 1.35, 95% CI: 1.1–1.6).18 To date, PCI has not been shown to reduce mortality in patients with stable coronary artery disease, however, if PMI can be reduced, these clinical outcomes would be expected to improve.
Although several therapeutic strategies have been suggested to reduce PMI, a procedural complication has been reported in about 5% to 30% of patients.192021 The most common mechanisms of PMI during PCI are side-branch occlusion and distal embolization.9 The main proposed mechanisms of side-branch occlusion are a snow plough effect (plaque shift) from the main vessel and carina shift,91122 with most side-branch occlusions occurring after post-stent dilation performed with high-pressure balloon inflation.12 Theoretically, PCB has a considerably lower chance of side-branch occlusion compared to a stent, because there is no plaque- or carina shift due to the absence of foreign objects such as stent struts. In this context, we recently demonstrated that using a PCB in main vessels with side-branches may be an option to avoid compromise of the side-branch ostium compared to PCI with DES using optical coherence tomography.23 Previously, we showed that PCB treatment of de novo coronary lesions of main vessels resulted in an increase in the SB ostial lumen area at 9-months follow-up.
Historically, stents reduce target vessel revascularization compared to plain old balloon angioplasty, however, a paradoxical increase in the incidence of PMI has been reported following stent implantation compared with that of plain old balloon angioplasty.2425 Compared with plain old balloon angioplasty in which the lumen expansion is predominantly due to plaque redistribution and plaque dissection, lumen enlargement after stenting involves a combination of plaque redistribution, plaque extrusion, vessel expansion, plaque compression, and plaque embolization.26 Stenting per se can be a cause, as small thrombi may form on their unendothelized surface and embolize distally.24 In addition, plaque disruption after stenting releases prothrombotic biofactors into the coronary circulation, leading to microvascular thrombosis, platelet activation, microcirculatory inflammation and oxidative stress, and no-reflow phenomenon.27
In this aspect, PCB treatment is an attractive therapeutic option and may have benefits over DES in further reducing the rate of PMI. Of note, PCB treatment is vastly superior to plain old balloon angioplasty for de novo coronary lesions because it can homogeneously deliver a drug to the vessel wall.28 In the present study, we demonstrated that PCB treatment for de novo coronary disease in patients who were unable to receive long-term dual antiplatelet therapy is promising in better tackling PMI than DES implantation. In terms of the safety and efficacy of PCB treatment for de novo coronary lesions, we have already shown that fractional flow reserve guided PCB treatment is safe and effective with good anatomical and physiological patency at follow-up.29 Therefore, we hope that PCB treatment will be used in a broader patient population with de novo coronary disease, thus reducing PMI post-PCI.
There are some limitations to the present study. Firstly, this study was an observational study with a small numbers of patients and lesions. Although there was a difference of side-branch occlusion between both groups, the number of side-branch occlusion was too small to assess the relationship with the risk of PMI. In addition, although the clinical outcomes were not included in the objectives of this study, we could not show the differences in clinical outcomes between PCB and DES in this study. Since this study was a single center trial with a small study population, the results cannot be applied to patients beyond the inclusion criteria and study protocol. Also, although this study performed a propensity score-matched analysis to adjust for potential confounding factors, it could not correct for unmeasured variables. Therefore, further studies in larger sample size with randomized study are required to confirm these findings. Fourthly, because we compared the first-generation DES to PCB, the results could be different from comparison results between other generation DES, but not first-generation and PCB. Finally, even though we applied the propensity score analysis in this study, we could not completely eliminate the selection bias in terms of excluding the cases of flow-limiting dissection after balloon angioplasty at pre-PCB procedure time. Nevertheless, we also excluded the failed PCI cases in the DES group.
In conclusion, treatment with a PCB on de novo coronary lesion might significantly reduce the risk of PMI compared to DES.
Figures and Tables
Table 1
Baseline Clinical Characteristics

Table 2
Procedural, Angiographic Characteristics and Incidence of PMI

PMI, peri-procedural myocardial infarction; PCB, paclitaxel-coated balloon; DES, drug eluting stent; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; SB, side-branch; MV, main vessel; QCA, quantitative coronary analysis.
Data are mean±SD or number (percentage).
Table 3
Predictors of PMI by Binomial Logistic Regression Analysis
References
1. Ioannidis JP, Karvouni E, Katritsis DG. Mortality risk conferred by small elevations of creatine kinase-MB isoenzyme after percutaneous coronary intervention. J Am Coll Cardiol. 2003; 42:1406–1411.

2. Bhatt DL, Topol EJ. Does creatinine kinase-MB elevation after percutaneous coronary intervention predict outcomes in 2005? Periprocedural cardiac enzyme elevation predicts adverse outcomes. Circulation. 2005; 112:906–915.

3. Cutlip DE, Kuntz RE. Does creatinine kinase-MB elevation after percutaneous coronary intervention predict outcomes in 2005? Cardiac enzyme elevation after successful percutaneous coronary intervention is not an independent predictor of adverse outcomes. Circulation. 2005; 112:916–922.

4. Kong TQ, Davidson CJ, Meyers SN, Tauke JT, Parker MA, Bonow RO. Prognostic implication of creatine kinase elevation following elective coronary artery interventions. JAMA. 1997; 277:461–466.

5. Saucedo JF, Mehran R, Dangas G, Hong MK, Lansky A, Kent KM, et al. Long-term clinical events following creatine kinase--myocardial band isoenzyme elevation after successful coronary stenting. J Am Coll Cardiol. 2000; 35:1134–1141.

6. Park DW, Kim YH, Yun SC, Ahn JM, Lee JY, Kim WJ, et al. Frequency, causes, predictors, and clinical significance of peri-procedural myocardial infarction following percutaneous coronary intervention. Eur Heart J. 2013; 34:1662–1669.

7. Chen SL, Zhang JJ, Ye F, Tian NL, Sheiban I, Jepson N, et al. Periprocedural myocardial infarction is associated with increased mortality in patients with coronary artery bifurcation lesions after implantation of a drug-eluting stent. Catheter Cardiovasc Interv. 2015; 85:Suppl 1. 696–705.

8. Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005; 294:1215–1223.

9. Babu GG, Walker JM, Yellon DM, Hausenloy DJ. Peri-procedural myocardial injury during percutaneous coronary intervention: an important target for cardioprotection. Eur Heart J. 2011; 32:23–31.

10. Prasad A, Herrmann J. Myocardial infarction due to percutaneous coronary intervention. N Engl J Med. 2011; 364:453–464.

11. Koo BK, Waseda K, Kang HJ, Kim HS, Nam CW, Hur SH, et al. Anatomic and functional evaluation of bifurcation lesions undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010; 3:113–119.

12. Aliabadi D, Tilli FV, Bowers TR, Benzuly KH, Safian RD, Goldstein JA, et al. Incidence and angiographic predictors of side branch occlusion following high-pressure intracoronary stenting. Am J Cardiol. 1997; 80:994–997.

13. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012; 60:1581–1598.

14. Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, et al. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010; 5:871–874.

15. Fuchs S, Kornowski R, Mehran R, Satler LF, Pichard AD, Kent KM, et al. Cardiac troponin I levels and clinical outcomes in patients with acute coronary syndromes: the potential role of early percutaneous revascularization. J Am Coll Cardiol. 1999; 34:1704–1710.

16. Cantor WJ, Newby LK, Christenson RH, Tuttle RH, Hasselblad V, Armstrong PW, et al. Prognostic significance of elevated troponin I after percutaneous coronary intervention. J Am Coll Cardiol. 2002; 39:1738–1744.

17. Fujii K, Carlier SG, Mintz GS, Kobayashi Y, Jacoboff D, Nierenberg H, et al. Creatine kinase-MB enzyme elevation and long-term clinical events after successful coronary stenting in lesions with ruptured plaque. Am J Cardiol. 2005; 95:355–359.

18. Nienhuis MB, Ottervanger JP, Bilo HJ, Dikkeschei BD, Zijlstra F. Prognostic value of troponin after elective percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv. 2008; 71:318–324.

19. Nallamothu BK, Bates ER. Periprocedural myocardial infarction and mortality: causality versus association. J Am Coll Cardiol. 2003; 42:1412–1414.
20. Lansky AJ, Stone GW. Periprocedural myocardial infarction: prevalence, prognosis, and prevention. Circ Cardiovasc Interv. 2010; 3:602–610.
21. Cuculi F, Lim CC, Banning AP. Periprocedural myocardial injury during elective percutaneous coronary intervention: is it important and how can it be prevented? Heart. 2010; 96:736–740.

22. Vetrovec GW, Cowley MJ, Wolfgang TC, Ducey KC. Effects of percutaneous transluminal coronary angioplasty on lesion-associated branches. Am Heart J. 1985; 109(5 Pt 1):921–925.

23. Her AY, Ann SH, Singh GB, Kim YH, Okamura T, Garg S, et al. Serial morphological changes of side-branch sstium after paclitaxel-coated balloon treatment of de novo coronary lesions of main vessels. Yonsei Med J. 2016; 57:606–613.

24. Kini A, Kini S, Marmur JD, Bertea T, Dangas G, Cocke TP, et al. Incidence and mechanism of creatine kinase-MB enzyme elevation after coronary intervention with different devices. Catheter Cardiovasc Interv. 1999; 48:123–129.

25. Dirschinger J, Kastrati A, Neumann FJ, Boekstegers P, Elezi S, Mehilli J, et al. Influence of balloon pressure during stent placement in native coronary arteries on early and late angiographic and clinical outcome: a randomized evaluation of high-pressure inflation. Circulation. 1999; 100:918–923.

26. Ahmed JM, Mintz GS, Weissman NJ, Lansky AJ, Pichard AD, Satler LF, et al. Mechanism of lumen enlargement during intracoronary stent implantation: an intravascular ultrasound study. Circulation. 2000; 102:7–10.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download