Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1Results of systemic venous sampling of fibroblast growth factor 23 (FGF23) in six patients. (A) Case 1, (B) Case 2, (C) Case 3, (D) Case 4, (E) Case 5, (F) Case 6. (a) Right (Rt.) jugular vein, (b) Left (Lt.) jugular vein, (c) Rt. subclavian vein, (d) Lt. subclavian vein, (e) superior vena cava, (f) inferior vena cava, (g) Rt. common iliac vein, (h) Lt. common iliac vein, (i) Rt. femoral vein, (j) Lt. femoral vein, (k) Rt. popliteal vein, (l) Lt. popliteal vein, (m) Rt. profunda femoris vein, (n) Lt. profunda femoris vein, (o) Rt. peroneal vein, and (p) Rt. anterior tibial vein. Numbers indicate FGF23 concentrations in pg/mL. ‘●’ means the finally confirmed causative tumor. |
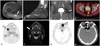 | Fig. 2Imaging data from patients with tumor-induced osteomalacia (TIO). (A) Magnetic resonance imaging (MRI) of the right thigh in case 1 shows a 2.7-cm oval mass within the right gluteus maximus. (B) MRI of the left foot in case 2 reveals a 4.2-cm lobulated mass in the posterior aspect of the ankle. (C) MRI of the left hip in case 3 shows a 0.8-cm, well-defined, round lesion in the left femoral head. (D) F-18 fluorodeoxyglucose positron emission tomography with computed tomography (FDG-PET/CT) in case 3 reveals mild FDG uptake in the left femoral head. (E) 68Ga-DOTATOC PET/CT in case 4 shows increased uptake in the right mandible. (F) MRI of the neck in case 4 with focal enhancement in the right mandible. (G) 68Ga-DOTATOC PET/CT in case 6 shows increased FDG uptake in the left ethmoid and nasal cavity. (H) Computed tomography of the paranasal sinus in case 6 reveals a 1.8-cm soft-tissue lesion in the left ethmoid sinus. Arrows indicate the tumors responsible for TIO. |
Table 1
Baseline Clinical Characteristics of the Six Patients with Suspected Tumor-Induced Osteomalacia

ALP, alkaline phosphatase; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; TRP, tubular reabsorption of phosphate; Tmp/GFR, maximal tubular reabsorption of phosphate corrected for glomerular filtration rate; FGF23, fibroblast growth factor 23; PHEX, phosphate-regulating endopeptidases homolog, X-linked; DMP1, dentin matrix protein 1; ND, not done.
*TRP=[1-(serum creatinine (mg/dL)×urinary P (mg/dL)]/[serum P (mg/dL)×urinary Cr (mg/dL)], †If TRP ≤0.86, then Tmp/GFR=TRP×serum P (mg/dL), If TRP >0.86, then Tmp/GFR=[0.3×TRP/(1-0.8×TRP)]×serum P (mg/dL).
Table 2
A Summary of Imaging Data for the Patients
FDG-PET/CT, F-18 fluorodeoxyglucose positron emission tomography with computed tomography; MRI, magnetic resonance imaging; Rt., right; Lt., left; H&N; head and neck; 68Ga-DOTATOC, 68Ga-DOTATOC positron emission tomography with computed tomography; PMTMCT, phosphaturic mesenchymal tumor mixed connective tissue variant; ND, not done.
−, not detected; +, detected.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download