Abstract
Purpose
Significant late-onset tricuspid regurgitation (TR) is unfortunately common after double valve replacement (DVR); however, its underlying factors remain undefined. We evaluated the effect of aortic patient-prosthesis mismatch (PPM) on late-onset TR and clinical outcomes after DVR.
Materials and Methods
Of the 2392 consecutive patients who underwent aortic valve replacement between January 1990 and May 2014 at our institution, we retrospectively studied 462 patients who underwent DVR (excluding concomitant tricuspid valvular annuloplasty or replacement). Survival and freedom from grade >3 TR were compared between PPM (n=152) and non-PPM (n=310) groups using the Kaplan-Meier method.
Results
Although the overall survival rates were similar between the two groups at 5 and 10 years (95%, 91% vs. 96%, 93%, p=0.412), grade >3 TR-free survival was significantly lower in the PPM group (98%, 91% vs. 99%, 95%, p=0.014). Small body-surface area, atrial fibrillation, PPM, and subaortic pannus were risk factors for TR progression. However, aortic prosthesis size and trans-valvular pressure gradient were not significant factors for either TR progression or overall survival.
Conclusion
Aortic PPM in DVR, regardless of mitral prosthesis size, was associated with late TR progression, but was not significantly correlated with overall survival. Therefore, we recommend careful echocardiographic follow-up for the early detection of TR progression in patients with aortic PPM in DVR.
The frequency of double valve replacement [DVR; concomitant mitral valve (MV) and aortic valve (AV) replacement] remains low (3% to 14%), compared with isolated valve surgery for aortic and mitral pathologies.12 Although a reduction in right-ventricular pressure or volume overload after concomitant left-sided valve surgery is expected to reduce the progression of tricuspid regurgitation (TR),3 TR does not always regress after adequate correction of underlying lesions45 and can even develop de novo postoperatively. The prevention of late TR is clinically important, because this condition adversely affects long-term mortality and morbidity, and surgical correction of TR after left-sided valve surgery is associated with disappointingly high mortality and morbidity.678
Significant late-onset TR is unfortunately common after DVR;910 however, the underlying factors remain undefined. Long-term follow-up studies have evaluated the incidence and risk factors associated with late TR development in organic TR patients or only in those with rheumatic valvular disease or mitral valvular lesions.511 Moreover, in the context of DVR, small-sized aortic prostheses are often chosen to prevent interference from mitral prostheses that are implanted earlier in the operation. In Asians especially, smaller aortic prostheses are commonly chosen in DVR cases for the previous reasons. Based on the hypothesis that the use of aortic prostheses of smaller size in DVR might induce severe patient-prosthesis mismatch (PPM) at the aortic position and adversely influence the right-heart hemodynamics, leading to TR development, this study investigated the effect of aortic prosthesis PPM on late-onset TR and clinical outcomes in DVR.
Among 2392 consecutive patients who underwent MV or AV surgery between January 1990 and May 2014 at Severance Cardiovascular Hospital, Yonsei University College of Medicine, 751 patients who had undergone DVR, with or without tricuspid valve surgery, were retrospectively reviewed. Excluding those who had undergone concomitant tricuspid repair or replacement surgery, coronary artery bypass grafting, and aorta surgery, a total of 462 patients were finally included in our study. Patients with unclear or missing operative records were also excluded.
The patients' hospital records and our surgical database were reviewed for the following: age at surgery, sex, body weight, heart rhythm, type of surgery, and history of prior cardiac surgery. A preoperative echocardiographic examination had been performed in all patients by a skilled and experienced cardiologist. The left-ventricular end-systolic diameter (mm) and left-ventricular end-diastolic diameter (mm), left-ventricular ejection fraction (LVEF, %), and valvular functions were compre-hensively evaluated. The etiologies of valvular disease were confirmed based on surgical observations and pathologic findings. Follow-up echocardiographic examinations were performed, focusing on the trans-aortic valve pressure gradient (PG, mm Hg). The study protocol was approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine. Individual patient consent was waived, because this study did not interfere with patient treatment and because the database was designed so that individual patients could not be identified. All baseline and clinical characteristics were obtained from the medical records of the patients.
Clinical and echocardiographic assessments were performed prior to DVR and 12–60 months after operation. The presence of TR and its severity were assessed using multiple transthoracic windows. The maximal TR jet area in any echocardiographic view was used to semi-quantitatively estimate the TR grade using a standard color Doppler technique. The TR grade was classified as none (0), trivial (1), mild (2), moderate (3), or severe (4) in each patient.12 To simplify the statistical analysis, significant TR was defined as moderate or severe in degree. The latest significant TR was regarded as the primary outcome of the follow-up period. The trans-aortic PG was measured by the continuous-wave Doppler method with sampling from multiple echocardiographic windows, using imaging and non-imaging Doppler probes. The LV dimensions were measured in the two-dimensional guided M-mode.13 The LVEF was determined by a combination of the Teichholz formula14 and visual assessment of LV function from multiple echocardiographic windows.
The effective orifice area (EOA, cm2) for each type and size of prosthesis was obtained from the literature or from the manufacturers,15 and the parameter of choice validated to identify PPM was the indexed EOA (IEOA), which is the EOA of the prosthesis being implanted indexed to the patient's body surface area (BSA). We classified PPM as not clinically significant (i.e., mild or absent) if the IEOA was >0.85 cm2/m2, as moderate if it was >0.65 and ≤0.85 cm2/m2, and as severe if it was ≤0.65 cm2/m2.16 To validate the cutoff IEOA value of 0.85, we constructed receiver operating characteristic curves, which indicated a cutoff value of IEOA=0.88 for inducing PPM (Fig. 1). To test the hypothesis that PPM is a strong correlation factor for TR progression, the patients were accordingly divided into PPM (IEOA ≤0.85; n=152) and non-PPM (IEOA >0.85; n=310) groups under the presumption that the cutoff IEOA value for defining PPM was reasonable (0.85≈0.88).
Continuous variables were expressed as mean±SD and categorical variables as percentages. The primary event outcomes were defined as significant TR (Gr. 3, 4) or repeat tricuspid valve surgery (valvuloplasty or replacement) after surgery, and the secondary event outcome was overall (cumulative) mortality. Significant-TR-free survival rates (%) were plotted using the Kaplan-Meier method, and comparisons were made between the groups with PPM and non-PPM aortic prosthetics using the log-rank test. Cox hazard regression analysis including all significant parameters identified by uni- and multivariate analysis was undertaken to identify parameters independently associated with the presence of significant TR and late mortality after surgery.
All reported p values were two-sided, and a value of p<0.05 was considered statistically significant. SPSS version 18.0 (IBM Corp., Armonk, NY, USA) and MedCalc (MedCalc Software, Ostend, Belgium) were used for the statistical analyses.
The baseline characteristics of the 462 study subjects are summarized in Table 1. The mean follow-up duration was 128±80 months. Preoperative electrocardiograms showed atrial fibrillation (AF) in 235 (51%) patients; 234 (50.6%) patients were female; the mean BSA was 1.7±0.4 (kg/m2); and 220 (47.6%) patients had New York Heart Association class 3 or 4 congestive heart failure. Mechanical and tissue DVR were performed in 414 patients and 48 patients, respectively. As a large portion of the patients had rheumatic pathology (85%), mechanical prosthesis was used in a majority of patients. The tissue valve replacement group showed a higher incidence of significant PPM, compared with the mechanical valve group (28% vs. 71%). Severe mitral stenosis (MS) and aortic stenosis (AS) patients comprised 330, while combined MS and regurgitation with AS accounted for 53 (Table 2). The mean aortic and mitral prosthesis sizes were 21.2±1.7 mm and 28.5±1.7 mm, respectively.
Aortic position PPM occurred in 152 patients (32.9%). The peak and mean values of the trans-aortic valvular pressure gradient (TAPG peak and mean, respectively; mm Hg) were significantly higher in the PPM group (42.3±20.9 vs. 30.5±17.3, p<0.001 and 24.4±12.8 vs. 17.3±10.6, p<0.001, respectively) at the latest postoperative follow-up. Subaortic pannus formation was observed in 58 patients (12.6%) [the presence of subaortic pannus was established by cardiac computed tomography in the case of high-transvalvular pressure gradient (TVPG) patients] and paravalvular leakage in any position (aortic or mitral) was observed in 23 patients (4.9%); 52 (11.3%) patients progressed to significant TR. PPM occurred most frequently with the 19-mm aortic prosthesis (75/152, 49.3%), regardless of mitral prosthesis size (Fig. 2).
Of the 462 study subjects, 61 patients died from any cause during follow-up (PPM: n=23, 15.1%; non-PPM: n=38, 12.3%). The overall survival rate was not significantly different between the groups (1, 5, and 10 years: 100%, 96%, 93% in PPM group vs. 99%, 95%, 91% in non-PPM group, p=0.41) (Fig. 3). Significant TR (grade 3 or 4) developed in 52 patients (11.3%) by the latest follow-up: 33 (21.7%) in the PPM group and 19 (6.2%) in the non-PPM group. The rate of freedom from significant TR was significantly different between the two groups: the PPM group showed significantly lower event-free survival than the non-PPM group according to Kaplan-Meier survival analysis (1, 5, and 10 years: 100%, 99%, 91% in PPM vs. 100%, 99%, 95% in non-PPM, p=0.014) (Fig. 4). To identify independent factors for the development of late-onset significant TR, we performed multivariate forward Cox hazard regression analysis using clinical and echocardiographic parameters, including age, sex, preoperative LV function (LVEF, %), presence of preoperative AF, aortic and mitral prosthesis size (mm), sub-aortic pannus formation, and TAPG at latest follow-up (mm Hg). This analysis identified the following as multivariate independent determinants of late TR development: small BSA [hazard ratio (HR), 0.31; 95% confidence interval (CI), 0.18–0.54; p=0.012], presence of preoperative AF (HR, 3.08; 95% CI, 1.34–7.09; p=0.008), PPM (IEOA ≤0.85 cm2/m2) (HR, 1.69; 95% CI, 0.91–3.17; p=0.046), and subaortic pannus formation (HR, 2.14, 95% CI, 1.06–4.31; p=0.033). In the analysis of overall survival, the meaningful multivariate determinants were age (HR, 1.05; 95% CI, 1.02–1.08; p=0.010) and subaortic pannus formation (HR, 4.12, 95% CI, 1.39–12.15; p=0.010) (Table 3).
In this study, late significant TR developed more often in patients with aortic PPM than in those without, regardless of aortic prosthesis size (from 19 mm to 25 mm). Despite the large number of published reports on the association between left-sided valvular surgery and TR progression, none had clearly established the possible underlying mechanisms of TR progression after DVR. The incidence of significant TR after left-heart valve surgery varies among reports, because of differences among study group compositions, follow-up durations, and definitions of significant TR,111718 with late severe TR reported in 14–43% of patients after MV surgery and in 7–27% of patients with left-sided valve surgery, including AV surgery alone. The incidence of significant TR after left-heart valve surgery also varies depending on the etiology of MV disease, from rheumatic to degenerative. Song, et al.19 reported that rheumatic etiology was strongly associated with the development of late significant TR. Pancarditis associated with the progression of rheumatic fever can result in increased right-ventricular susceptibility both to subclinical damage and to pro-gressive dysfunction and geometric change after exposure to hemodynamic changes provoked by left-sided valvular lesions.20 In this study, despite the high proportion of patients (83%) with rheumatic valvular pathology, this factor showed no association with late significant TR progression in univariate analysis (HR, 1.91, 95% CI, 0.46–1.87; p=0.372).
The sex difference in the incidence of late TR also needs further clarification. In a study with long-term follow-up (11.3 years) of 65 patients with rheumatic heart disease and significant preoperative organic TR, the incidence of late TR was 67%, and female sex was an independent predictor of TR development.5 Song, et al.19 also posited that female sex was an independent predictor of TR progression because of the confounding effect of a relatively high prevalence of rheumatic involvement of the MV. These results may suggest that female sex, as opposed to male sex, is associated with the presence of a small aorta or AV annulus, which results in the choice of a small aortic prosthesis. However, in the present study, female sex was only a predictor of late TR progression in the univariate analysis and lost its significance as a multivariate risk factor. Only small BSA, regardless of sex, was a strong predictor for late TR progression. In the context of DVR, the aortic prosthesis size can also be affected by an implanted mitral prosthesis, and in fact, we selected smaller-sized prostheses to aid safe implantation in the aortic position. Therefore, small BSA, regardless of sex, might be a key factor for the selection of the aortic prosthesis in DVR.
Preoperative AF is also a well-known significant risk factor for late TR progression. A previous study found a 16% incidence of late significant TR during an 8.2-year follow-up of 174 patients after MV surgery, with AF as an independent predictor.11 The potential association between AF and late TR was also supported by the finding that a concomitant maze procedure was beneficial for the prevention of late TR.21 In the present study, there were 235 cases with preoperative AF, which has also been documented as a strong risk factor for late TR progression (HR, 5.31, 95% CI, 2.02–11.12; p<0.001).
The TVPG is also regarded as an important causal factor for late TR progression. High TVPG at an aortic position can induce left-ventricular hypertrophic changes, which appear to be associated with gradually increasing left-atrial pressure, eventually resulting in right-heart structural changes manifesting as TR. High TVPG at an aortic position may also underlie pannus formation, which has been linked to inflammatory re-actions against a foreign body or to turbulent transvalvular blood flow. However, in the present study, high TVPG (both peak and mean) was not itself a predicting factor (HR, 1.03, 95% CI, 0.96–1.67; p=0.391), although resulting pannus formation was a strong predictor of late-onset TR (HR, 4.86, 95% CI, 2.13–11.12; p< 0.001). These results suggest that high TVPG may not always be correlated with pannus formation and that high TVPG without pannus might not induce late TR. This might be related with the nature of PPM and pannus formation. Though pannus formation is a progressive process, it might not be an issue before it induces significant narrowing. When pannus creates a certain level of narrowing, the acute pressure load can have a sudden impact on the right side of the heart and TR. Unlike this, the increase of TVPG from simple valvular degeneration is a gradual process with sufficient time for the right heart to adapt to the increased pressure. Yoshikawa, et al.22 suggested that small Asian female patients might have a greater tendency to form pannus than Western patients, but did not explore an association with late TR progression.
In terms of the effects of small-sized aortic prostheses on late-onset TR, Jeong suggested that a high TVPG might be related to the progression to late TR in 132 patients (mean age 54±13 years) who underwent AVR using ATS valves.23 However, that study only included ATS valves and a relatively small study population, and did not evaluate subaortic pannus. In our study, TVPG was not a multivariate predictor of late TR, although it was significant in univariate analysis.
Cox hazard analysis of aortic PPM showed a statistically strong correlation with late TR progression (HR, 1.95, 95% CI, 1.12–3.40; p=0.029). From this result, we suggest that taking steps to avoid aortic PPM where possible would reduce the risk of TR progression. In our study, smaller aortic prostheses were associated with greater PPM incidence, and most cases of PPM occurred with 19-mm-sized prostheses (Fig. 2). Therefore, a careful check for PPM following the manufacturer's instructions, especially when a small size (e.g., 19 mm) is selected, would be an important step during DVR to minimize the risk of late TR progression, although there was no significant correlation herein between PPM and the cumulative survival rate (HR, 0.80, 95% CI, 0.48–1.46; p=0.412). Size itself did not show significant correlation with TR incidence, although a rather larger size showed more TR onset in our analysis using scatter grams (Supplementary Fig. 1, only online). Also in our results, tissue valve replacement showed a higher incidence of significant PPM than mechanical valves (28% vs. 71%), and it might be related with the sex of patients, as tissue valves are preferred for young female patients with smaller BSA than male patients.
Several limitations of this study need to be acknowledged. First, the study is limited by its retrospective nature. Second, the tricuspid annulus size was not quantified in all cases. Pre-existing dilatation of the tricuspid annulus in the absence of TR may be associated with a higher rate of progression to TR, compared with the normal-annulus population. Third, mitral position PPM was not considered seriously in this analysis. Actually, the effect of mitral PPM on late survival is uncertain, and estimates of EOA can vary largely among different methods of calculation,24 which could have some effect on results. Finally, the pathology of most patients was rheumatic; despite its statistical insignificance as a risk factor according to univariate analysis, the progression of rheumatic disease might be considerably different in the study population, compared with the general population. Given such differences, the progression of TR may have been affected by rheumatic involvement in each case.
In conclusion, this study identified risk factors (small BSA, AF, aortic PPM, and pannus at the aortic position) associated with progression to significant TR after left-sided DVR. The choice of a small aortic prosthesis (≤19 mm) in DVR was associated with a higher rate of PPM, which in turn was strongly correlated with late TR progression. Therefore, careful follow-up by echocardiography for the detection of TR progression is important in patients with small aortic prosthesis implantation in the context of DVR.
Figures and Tables
 | Fig. 1ROC curve of aortic prosthesis size analysis to obtain the cutoff size value for inducing PPM. IEOA, indexed effective orifice area; TR, tricuspid regurgitation; ROC, receiver operating characteristic; PPM, patientprosthesis mismatch. |
 | Fig. 2PPM incidence according to aortic prosthesis size. PPM, patientprosthesis mismatch; IEOA, indexed effective orifice area. |
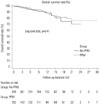 | Fig. 3Overall cumulative survival rates for PPM and non-PPM groups. PPM, patient-prosthesis mismatch. |
 | Fig. 4TR-progression-free survival rates for PPM and non-PPM groups. TR, tricuspid regurgitation; PPM, patient-prosthesis mismatch. |
Table 1
Patient Pre- and Post-Operative Data

EF, ejection fraction; BSA, body surface area; AF, atrial fibrillation; NYHA, New York Heart Association; TAPG, trans-aortic valvular pressure gradient; LVMI, left ventricular mass index; LV, left ventricular; EOA, effective orifice area; RVSP, right ventricular systolic pressure.
Data are presented as a mean±SD or n (%).
Table 2
Preoperative Underlying Valve Diseases at Time of Surgery

| MV disease | AV disease | Total | ||
|---|---|---|---|---|
| AS | AR | ASR | ||
| MS | 330 | 2 | 15 | 347 |
| MR | 21 | 13 | 11 | 45 |
| MSR | 53 | 1 | 16 | 70 |
| Total | 404 | 16 | 42 | 462 |
Table 3
Cox Hazard Regression Analysis for Late TR Progression and Overall Survival in the DVR Population

ACKNOWLEDGEMENTS
This study was supported by a faculty research grant from Yonsei University College of Medicine for 6-2014-0157.
References
1. Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of The Society of Thoracic Surgeons. Ann Thorac Surg. 1999; 67:943–951.

2. Hannan EL, Racz MJ, Jones RH, Gold JP, Ryan TJ, Hafner JP, et al. Predictors of mortality for patients undergoing cardiac valve replacements in New York State. Ann Thorac Surg. 2000; 70:1212–1218.

3. Hannoush H, Fawzy ME, Stefadouros M, Moursi M, Chaudhary MA, Dunn B. Regression of significant tricuspid regurgitation after mitral balloon valvotomy for severe mitral stenosis. Am Heart J. 2004; 148:865–870.

4. McGrath LB, Gonzalez-Lavin L, Bailey BM, Grunkemeier GL, Fernandez J, Laub GW. Tricuspid valve operations in 530 patients. Twenty-five-year assessment of early and late phase events. J Thorac Cardiovasc Surg. 1990; 99:124–133.
5. Porter A, Shapira Y, Wurzel M, Sulkes J, Vaturi M, Adler Y, et al. Tricuspid regurgitation late after mitral valve replacement: clinical and echocardiographic evaluation. J Heart Valve Dis. 1999; 8:57–62.
6. Groves PH, Lewis NP, Ikram S, Maire R, Hall RJ. Reduced exercise capacity in patients with tricuspid regurgitation after successful mitral valve replacement for rheumatic mitral valve disease. Br Heart J. 1991; 66:295–301.

7. Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002; 144:524–529.

8. King RM, Schaff HV, Danielson GK, Gersh BJ, Orszulak TA, Piehler JM, et al. Surgery for tricuspid regurgitation late after mitral valve replacement. Circulation. 1984; 70(3 Pt 2):I193–I197.
9. Czer LS, Maurer G, Bolger A, DeRobertis M, Kleinman J, Gray RJ, et al. Tricuspid valve repair. Operative and follow-up evaluation by Doppler color flow mapping. J Thorac Cardiovasc Surg. 1989; 98:101–110.
10. Goldman ME, Guarino T, Fuster V, Mindich B. The necessity for tricuspid valve repair can be determined intraoperatively by two-dimensional echocardiography. J Thorac Cardiovasc Surg. 1987; 94:542–550.

11. Matsuyama K, Matsumoto M, Sugita T, Nishizawa J, Tokuda Y, Matsuo T. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg. 2003; 75:1826–1828.

12. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003; 16:777–802.

13. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463.

14. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976; 37:7–11.

15. Dumesnil JG, Honos GN, Lemieux M, Beauchemin J. Validation and applications of indexed aortic prosthetic valve areas calculated by Doppler echocardiography. J Am Coll Cardiol. 1990; 16:637–643.

16. Blais C, Dumesnil JG, Baillot R, Simard S, Doyle D, Pibarot P. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation. 2003; 108:983–988.

17. Kwak JJ, Kim YJ, Kim MK, Kim HK, Park JS, Kim KH, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. 2008; 155:732–737.

18. Izumi C, Iga K, Konishi T. Progression of isolated tricuspid regurgitation late after mitral valve surgery for rheumatic mitral valve disease. J Heart Valve Dis. 2002; 11:353–356.
19. Song H, Kim MJ, Chung CH, Choo SJ, Song MG, Song JM, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart. 2009; 95:931–936.

20. Asano K, Washio M, Eguchi S. Results of mitral valve replacement, with special reference to the functional tricuspid insufficiency. Jpn Heart J. 1971; 12:507–516.

21. Kim HK, Kim YJ, Kim KI, Jo SH, Kim KB, Ahn H, et al. Impact of the maze operation combined with left-sided valve surgery on the change in tricuspid regurgitation over time. Circulation. 2005; 112:I14–I19.

22. Yoshikawa K, Fukunaga S, Arinaga K, Hori H, Nakamura E, Ueda T, et al. Long-term results of aortic valve replacement with a small St. Jude medical valve in Japanese patients. Ann Thorac Surg. 2008; 85:1303–1308.

23. Jeong DS, Kim WS, Sung K, Yang JH, Jun TG, Lee YT, et al. Long-term hemodynamic performance of ATS valves in the aortic position: impact on the progression of late tricuspid regurgitation. J Heart Valve Dis. 2013; 22:794–803.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download