MATERIALS AND METHODS
Study population
A total of 10177 consecutive patients presented with typical or atypical chest pain and underwent coronary angiography (CAG) from November 2004 to May 2014 at the Cardiovascular Center of Korea University Guro Hospital, Seoul, South Korea. Among these, 3758 patients [51.8% men; age (mean±SD) 57.0±11.5 years] presented with typical or atypical chest pain and with coronary artery stenosis less than 50% on quantitative coronary angiography (QCA) and underwent the Ach provocation test. First line therapy for vasospastic angina included both nitrates and CCB. Nitrate therapy was used upon the first description of vasospastic angina and was reduced or discontinued when the patients'angina episodes were markedly decreased or nitroglycerin intolerable patients complained of refractory headaches or dizziness. Diltiazem was used with a 30-mg dose, because in some patients with relatively low blood pressure, side effects started at a lower dose of 30 mg per day. Once patient's symptom stabilized, the dose of diltiazem was increased to 180 mg per day in the follow-up period, and most maintenance doses were slow-releasing 180 mg per day or 90 mg per day. Patients were excluded if they had any prior coronary artery bypass graft (CABG), prior percutaneous coronary intervention (PCI), prior cerebrovascular disease, advanced heart failure (New York Heart Association class III or IV) or serum creatinine ≥2 mg/dL, since these conditions could be major causes of adverse cardiovascular events and could serve as a bias in the result of the study. Finally 2741 patients were enrolled and divided into two groups: the diltiazem group (diltiazem only; n=842) and the dual group (diltiazem and nitrate; n=1899). To adjust for potential confounders, a propensity score matching (PSM) analysis was performed using logistic regression.
Study definition
Significant CAS was defined as a luminal narrowing of more than 70% during Ach provocation test with or without ischemic electrocardiogram (ECG) changes or chest pain. Deaths were regarded as cardiac death unless non-cardiac death was confirmed. Repeat CAG was performed in patients who presented with recurrent angina despite treatment with adequate anti-angina medication at least for 6 months since the first CAG. In this case, the physician assumed that the CAS may have worsened or that new coronary artery disease (CAD) may have developed. Major adverse cardiovascular events (MACE) were defined as the composite of total death, de novo MI, and revascularization including PCI and CABG.
Acetylcholine provocation test
The methods of the Ach provocation test has been described previously.
45 In brief, CAG was performed to confirm the presence of significant CAD. All vasodilators and vasoconstrictors, such as nitrates, CCB, beta blockers, nicorandil, and molsidomine, were discontinued at least 72 hours before CAG. Incremental doses of 20 (A1), 50 (A2), and 100 (A3) µg/min of Ach were administered into the left coronary artery over a 1-minute period with 5-minute intervals up to the maximal tolerated dose under continuous monitoring of ECG and blood pressure. Routine Ach provocation testing of the right coronary artery was not conducted due to safety issues associated with a higher incidence of advanced atrioventricular (AV) block. At the end of the test, intracoronary injection of 0.2 mg of nitroglycerine was done after completing the Ach provocation test, and then CAG was done after 2 minutes. If focal or diffuse significant vasoconstriction (>70%) of the coronary arteries was induced at any dose, Ach infusion was stopped. End-systolic images for each segment of the left coronary artery were chosen according to the corresponding points on the electrocardiographic trace (QRS onset or end of T wave) and analyzed using the proper QCA system of the catheterization laboratory (FD-20, Phillips, Amsterdam, the Netherlands). The coronary artery diameters were measured by QCA before and after administration of Ach at the site that showed the greatest changes following drug administration. Reference vessel diameters were measured at the proximal and distal portions of each artery. The mean reference vessel diameter was used to assess diameter narrowing by QCA. Myocardial bridge was defined as the characteristic phasic systolic compression of the coronary artery with a decrease of more than 30% in diameter on the angiogram after intracoronary nitroglycerin infusion, mostly in anterior-posterior cranial or right anterior oblique cranial projections. Multi-vessel spasm was defined with significant CAS of more than two major epicardial arteries. Diffuse CAS was defined as significant CAS with the site length of more than 20 mm.
5 Baseline spasm was defined as focal or diffuse narrowing of greater than 30% in baseline CAG, compared to the reference vessel diameter after nitroglycerin administration into intracoronary route.
Statistical analysis
All the statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean±SD between groups, and differences were evaluated by Student's t-test. Discrete variables were expressed as counts and percentages, and differences were analyzed with χ2 (or Fisher's exact) test between groups as appropriate. Multivariate logistic regression analysis, which included baseline confounding factors, was used for assessing the independent impact factors. A two-tailed p value of <0.05 was considered to be statistically significant. To adjust for potential confounders, propensity score analysis was performed using the logistic regression model. We tested all available variables that could be of potential relevance: age, male, cardiovascular risk factors (hypertension, diabetes, dyslipidemia, current smokers, current alcoholics, and coronary fixed lesion) and myocardial bridge. The logistic model by which the propensity score was estimated showed good predictive value (C statistic=0.708). Patients treated with diltiazem alone were then 1-to-1 matched to the patients treated with diltiazem and nitrate on the propensity scores with the nearest available pair matching method. Subjects were matched with a caliper width equal to 0.01. The procedure yielded 512 well-matched pairs. Various clinical outcomes at 5-year were estimated with the Kaplan-Meier method, and differences between groups were compared with the log-rank test. Also, multivariate cox-regression analysis adjusted with following variables was performed to determine the impact of the use of diltiazem alone versus the use of diltiazem with nitrate on the incidence of 5-year clinical outcomes. The following factors were co-analyzed in multivariable analysis: age, gender, diabetes, dyslipidemia, hypertension, current smoking, current alcoholics, aspirin, trimetazidine, beta-blockers, diuretics, angiotensin receptor blocker, angiotensin converting enzyme inhibitor, statins, and myocardial bridge.
Study end points
The incidence of cumulative individual and composite clinical end points, such as mortality (cardiac and non-cardiac death), PCI, MI, cerebrovascular accident, repeat CAG, and MACE, were evaluated for up to 5 years. In this study, all patients completed a 5-year clinical follow up by face-to-face interviews, phone calls, or chart review. The mean follow-up period was 1825 days, and we could follow up clinical data from all the enrolled patients.
Go to :

RESULTS
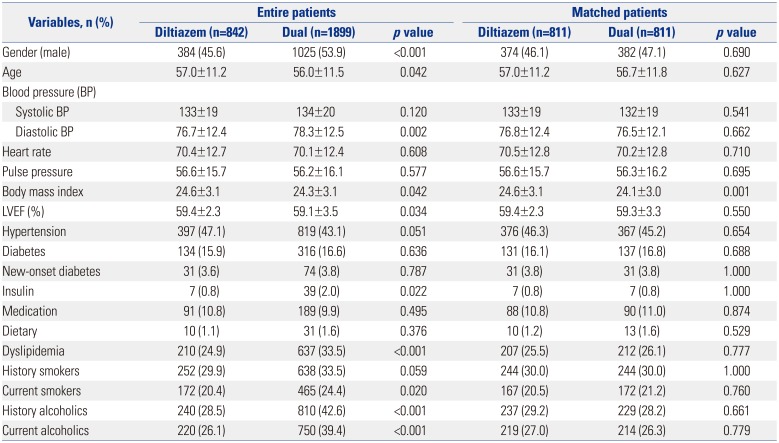
The baseline clinical characteristics of patients are shown in
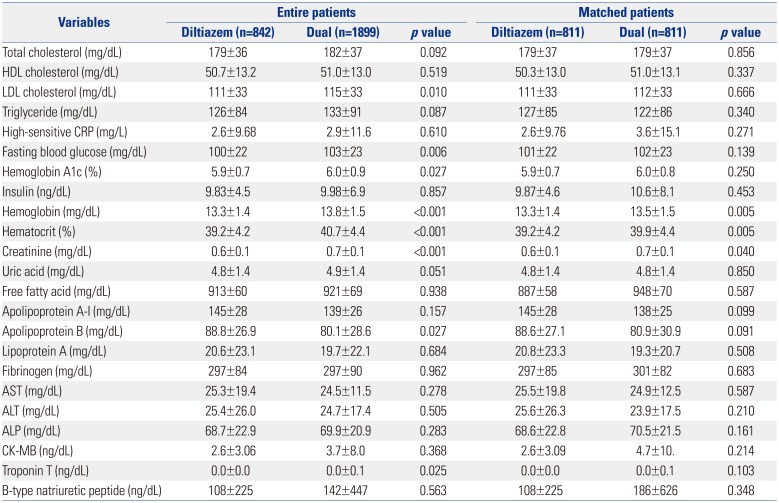
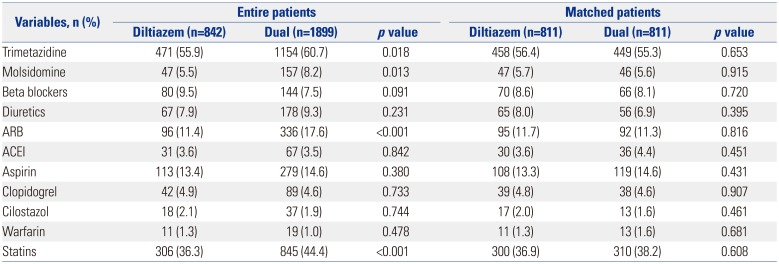
Table 1. A total of 2741 patients were finally enrolled. Among the patients with significant positive response to the Ach provocation test, the patients treated with the diltiazem only regimen accounted for 30.7% (842/2741), and the patients treated with diltiazem and nitrate combination comprised 69.3% (1899/2741). Before PSM, baseline characteristics showed that the diltiazem alone group were of older age and had greater body mass index (BMI), higher left ventricular ejection fraction, less male subjects, lower lipid levels, less current smokers, and less alcohol consumers compared with the dual group. However, there were no significant differences in hypertension, diabetes mellitus, or history of smoking between the two groups. With regard to medications, the diltiazem group and the diltiazem and nitrated group were equally treated with beta blockers, angiotensin converting enzyme inhibitors, aspirin, clopidogrel, cilostazol, and warfarin. However, the combined treatment group was treated with more trimetazidine, molsidomine, angiotensin receptor blockers, and statins, compared to the diltiazem only group. However, after PSM, the baseline clinical characteristics and the use of other medications was well balanced between the two groups (
Table 1,
2,
3).
Table 1
Baseline Clinical Characteristics between the Two Groups
|
Variables, n (%) |
Entire patients |
Matched patients |
|
Diltiazem (n=842) |
Dual (n=1899) |
p value |
Diltiazem (n=811) |
Dual (n=811) |
p value |
|
Gender (male) |
384 (45.6) |
1025 (53.9) |
<0.001 |
374 (46.1) |
382 (47.1) |
0.690 |
|
Age |
57.0±11.2 |
56.0±11.5 |
0.042 |
57.0±11.2 |
56.7±11.8 |
0.627 |
|
Blood pressure (BP) |
|
|
|
|
|
|
|
Systolic BP |
133±19 |
134±20 |
0.120 |
133±19 |
132±19 |
0.541 |
|
Diastolic BP |
76.7±12.4 |
78.3±12.5 |
0.002 |
76.8±12.4 |
76.5±12.1 |
0.662 |
|
Heart rate |
70.4±12.7 |
70.1±12.4 |
0.608 |
70.5±12.8 |
70.2±12.8 |
0.710 |
|
Pulse pressure |
56.6±15.7 |
56.2±16.1 |
0.577 |
56.6±15.7 |
56.3±16.2 |
0.695 |
|
Body mass index |
24.6±3.1 |
24.3±3.1 |
0.042 |
24.6±3.1 |
24.1±3.0 |
0.001 |
|
LVEF (%) |
59.4±2.3 |
59.1±3.5 |
0.034 |
59.4±2.3 |
59.3±3.3 |
0.550 |
|
Hypertension |
397 (47.1) |
819 (43.1) |
0.051 |
376 (46.3) |
367 (45.2) |
0.654 |
|
Diabetes |
134 (15.9) |
316 (16.6) |
0.636 |
131 (16.1) |
137 (16.8) |
0.688 |
|
New-onset diabetes |
31 (3.6) |
74 (3.8) |
0.787 |
31 (3.8) |
31 (3.8) |
1.000 |
|
Insulin |
7 (0.8) |
39 (2.0) |
0.022 |
7 (0.8) |
7 (0.8) |
1.000 |
|
Medication |
91 (10.8) |
189 (9.9) |
0.495 |
88 (10.8) |
90 (11.0) |
0.874 |
|
Dietary |
10 (1.1) |
31 (1.6) |
0.376 |
10 (1.2) |
13 (1.6) |
0.529 |
|
Dyslipidemia |
210 (24.9) |
637 (33.5) |
<0.001 |
207 (25.5) |
212 (26.1) |
0.777 |
|
History smokers |
252 (29.9) |
638 (33.5) |
0.059 |
244 (30.0) |
244 (30.0) |
1.000 |
|
Current smokers |
172 (20.4) |
465 (24.4) |
0.020 |
167 (20.5) |
172 (21.2) |
0.760 |
|
History alcoholics |
240 (28.5) |
810 (42.6) |
<0.001 |
237 (29.2) |
229 (28.2) |
0.661 |
|
Current alcoholics |
220 (26.1) |
750 (39.4) |
<0.001 |
219 (27.0) |
214 (26.3) |
0.779 |

Table 2
Baseline Laboratory Findings between the Two Groups
|
Variables |
Entire patients |
Matched patients |
|
Diltiazem (n=842) |
Dual (n=1899) |
p value |
Diltiazem (n=811) |
Dual (n=811) |
p value |
|
Total cholesterol (mg/dL) |
179±36 |
182±37 |
0.092 |
179±37 |
179±37 |
0.856 |
|
HDL cholesterol (mg/dL) |
50.7±13.2 |
51.0±13.0 |
0.519 |
50.3±13.0 |
51.0±13.1 |
0.337 |
|
LDL cholesterol (mg/dL) |
111±33 |
115±33 |
0.010 |
111±33 |
112±33 |
0.666 |
|
Triglyceride (mg/dL) |
126±84 |
133±91 |
0.087 |
127±85 |
122±86 |
0.340 |
|
High-sensitive CRP (mg/L) |
2.6±9.68 |
2.9±11.6 |
0.610 |
2.6±9.76 |
3.6±15.1 |
0.271 |
|
Fasting blood glucose (mg/dL) |
100±22 |
103±23 |
0.006 |
101±22 |
102±23 |
0.139 |
|
Hemoglobin A1c (%) |
5.9±0.7 |
6.0±0.9 |
0.027 |
5.9±0.7 |
6.0±0.8 |
0.250 |
|
Insulin (ng/dL) |
9.83±4.5 |
9.98±6.9 |
0.857 |
9.87±4.6 |
10.6±8.1 |
0.453 |
|
Hemoglobin (mg/dL) |
13.3±1.4 |
13.8±1.5 |
<0.001 |
13.3±1.4 |
13.5±1.5 |
0.005 |
|
Hematocrit (%) |
39.2±4.2 |
40.7±4.4 |
<0.001 |
39.2±4.2 |
39.9±4.4 |
0.005 |
|
Creatinine (mg/dL) |
0.6±0.1 |
0.7±0.1 |
<0.001 |
0.6±0.1 |
0.7±0.1 |
0.040 |
|
Uric acid (mg/dL) |
4.8±1.4 |
4.9±1.4 |
0.051 |
4.8±1.4 |
4.8±1.4 |
0.850 |
|
Free fatty acid (mg/dL) |
913±60 |
921±69 |
0.938 |
887±58 |
948±70 |
0.587 |
|
Apolipoprotein A-I (mg/dL) |
145±28 |
139±26 |
0.157 |
145±28 |
138±25 |
0.099 |
|
Apolipoprotein B (mg/dL) |
88.8±26.9 |
80.1±28.6 |
0.027 |
88.6±27.1 |
80.9±30.9 |
0.091 |
|
Lipoprotein A (mg/dL) |
20.6±23.1 |
19.7±22.1 |
0.684 |
20.8±23.3 |
19.3±20.7 |
0.508 |
|
Fibrinogen (mg/dL) |
297±84 |
297±90 |
0.962 |
297±85 |
301±82 |
0.683 |
|
AST (mg/dL) |
25.3±19.4 |
24.5±11.5 |
0.278 |
25.5±19.8 |
24.9±12.5 |
0.587 |
|
ALT (mg/dL) |
25.4±26.0 |
24.7±17.4 |
0.505 |
25.6±26.3 |
23.9±17.5 |
0.210 |
|
ALP (mg/dL) |
68.7±22.9 |
69.9±20.9 |
0.283 |
68.6±22.8 |
70.5±21.5 |
0.161 |
|
CK-MB (ng/dL) |
2.6±3.06 |
3.7±8.0 |
0.368 |
2.6±3.09 |
4.7±10. |
0.214 |
|
Troponin T (ng/dL) |
0.0±0.0 |
0.0±0.1 |
0.025 |
0.0±0.0 |
0.0±0.1 |
0.103 |
|
B-type natriuretic peptide (ng/dL) |
108±225 |
142±447 |
0.563 |
108±225 |
186±626 |
0.348 |

Table 3
Medications between the Two Groups
|
Variables, n (%) |
Entire patients |
Matched patients |
|
Diltiazem (n=842) |
Dual (n=1899) |
p value |
Diltiazem (n=811) |
Dual (n=811) |
p value |
|
Trimetazidine |
471 (55.9) |
1154 (60.7) |
0.018 |
458 (56.4) |
449 (55.3) |
0.653 |
|
Molsidomine |
47 (5.5) |
157 (8.2) |
0.013 |
47 (5.7) |
46 (5.6) |
0.915 |
|
Beta blockers |
80 (9.5) |
144 (7.5) |
0.091 |
70 (8.6) |
66 (8.1) |
0.720 |
|
Diuretics |
67 (7.9) |
178 (9.3) |
0.231 |
65 (8.0) |
56 (6.9) |
0.395 |
|
ARB |
96 (11.4) |
336 (17.6) |
<0.001 |
95 (11.7) |
92 (11.3) |
0.816 |
|
ACEI |
31 (3.6) |
67 (3.5) |
0.842 |
30 (3.6) |
36 (4.4) |
0.451 |
|
Aspirin |
113 (13.4) |
279 (14.6) |
0.380 |
108 (13.3) |
119 (14.6) |
0.431 |
|
Clopidogrel |
42 (4.9) |
89 (4.6) |
0.733 |
39 (4.8) |
38 (4.6) |
0.907 |
|
Cilostazol |
18 (2.1) |
37 (1.9) |
0.744 |
17 (2.0) |
13 (1.6) |
0.461 |
|
Warfarin |
11 (1.3) |
19 (1.0) |
0.478 |
11 (1.3) |
13 (1.6) |
0.681 |
|
Statins |
306 (36.3) |
845 (44.4) |
<0.001 |
300 (36.9) |
310 (38.2) |
0.608 |

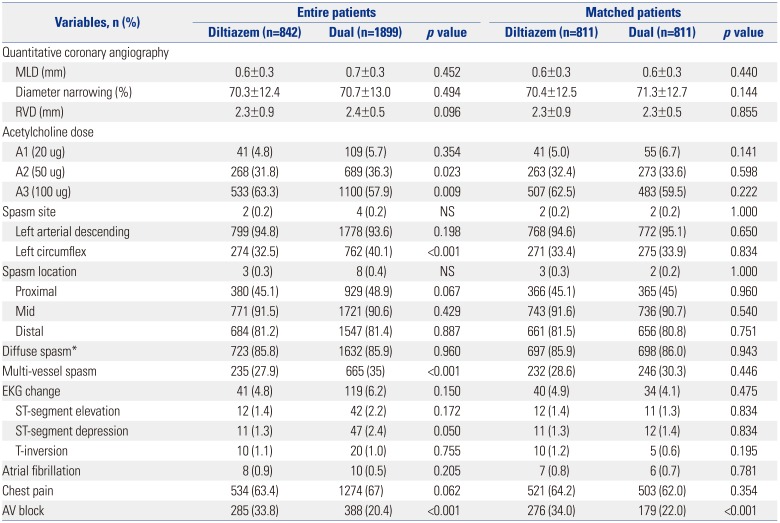
We evaluated the various clinical and angiographic parameters during the Ach provocation test, including the incidence of ischemic chest pain, ischemic ECG changes, response to Ach dose, and CAS extent between the two groups. The results are shown in
Table 4. Before adjusting for PSM, the angiographic and clinical characteristics of both groups were similar in terms of narrowing diameter during the Ach provocation test, reference vessel diameter after nitroglycerin injection, left anterior descending spasm, spasm locations, diffuse spasm, ECG changes, and chest pain. The combined treatment group had more multi-vessel spasms and less AV block, compared with the diltiazem alone group (
Table 4). Nevertheless, after PSM, the angiographic characteristics were similar between the two groups, except that the patients with dual regimen therapy had lower incidence of AV block.
Table 4
Angiographic Characteristics between the Two Groups
|
Variables, n (%) |
Entire patients |
Matched patients |
|
Diltiazem (n=842) |
Dual (n=1899) |
p value |
Diltiazem (n=811) |
Dual (n=811) |
p value |
|
Quantitative coronary angiography |
|
|
|
|
|
|
|
MLD (mm) |
0.6±0.3 |
0.7±0.3 |
0.452 |
0.6±0.3 |
0.6±0.3 |
0.440 |
|
Diameter narrowing (%) |
70.3±12.4 |
70.7±13.0 |
0.494 |
70.4±12.5 |
71.3±12.7 |
0.144 |
|
RVD (mm) |
2.3±0.9 |
2.4±0.5 |
0.096 |
2.3±0.9 |
2.3±0.5 |
0.855 |
|
Acetylcholine dose |
|
|
|
|
|
|
|
A1 (20 ug) |
41 (4.8) |
109 (5.7) |
0.354 |
41 (5.0) |
55 (6.7) |
0.141 |
|
A2 (50 ug) |
268 (31.8) |
689 (36.3) |
0.023 |
263 (32.4) |
273 (33.6) |
0.598 |
|
A3 (100 ug) |
533 (63.3) |
1100 (57.9) |
0.009 |
507 (62.5) |
483 (59.5) |
0.222 |
|
Spasm site |
2 (0.2) |
4 (0.2) |
NS |
2 (0.2) |
2 (0.2) |
1.000 |
|
Left arterial descending |
799 (94.8) |
1778 (93.6) |
0.198 |
768 (94.6) |
772 (95.1) |
0.650 |
|
Left circumflex |
274 (32.5) |
762 (40.1) |
<0.001 |
271 (33.4) |
275 (33.9) |
0.834 |
|
Spasm location |
3 (0.3) |
8 (0.4) |
NS |
3 (0.3) |
2 (0.2) |
1.000 |
|
Proximal |
380 (45.1) |
929 (48.9) |
0.067 |
366 (45.1) |
365 (45) |
0.960 |
|
Mid |
771 (91.5) |
1721 (90.6) |
0.429 |
743 (91.6) |
736 (90.7) |
0.540 |
|
Distal |
684 (81.2) |
1547 (81.4) |
0.887 |
661 (81.5) |
656 (80.8) |
0.751 |
|
Diffuse spasm*
|
723 (85.8) |
1632 (85.9) |
0.960 |
697 (85.9) |
698 (86.0) |
0.943 |
|
Multi-vessel spasm |
235 (27.9) |
665 (35) |
<0.001 |
232 (28.6) |
246 (30.3) |
0.446 |
|
EKG change |
41 (4.8) |
119 (6.2) |
0.150 |
40 (4.9) |
34 (4.1) |
0.475 |
|
ST-segment elevation |
12 (1.4) |
42 (2.2) |
0.172 |
12 (1.4) |
11 (1.3) |
0.834 |
|
ST-segment depression |
11 (1.3) |
47 (2.4) |
0.050 |
11 (1.3) |
12 (1.4) |
0.834 |
|
T-inversion |
10 (1.1) |
20 (1.0) |
0.755 |
10 (1.2) |
5 (0.6) |
0.195 |
|
Atrial fibrillation |
8 (0.9) |
10 (0.5) |
0.205 |
7 (0.8) |
6 (0.7) |
0.781 |
|
Chest pain |
534 (63.4) |
1274 (67) |
0.062 |
521 (64.2) |
503 (62.0) |
0.354 |
|
AV block |
285 (33.8) |
388 (20.4) |
<0.001 |
276 (34.0) |
179 (22.0) |
<0.001 |

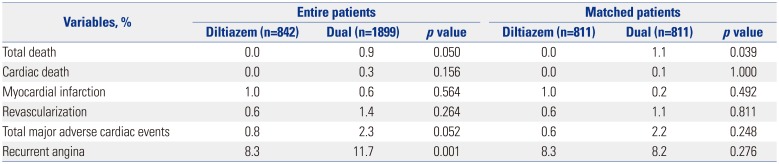
We evaluated the cardiovascular outcomes up to 5 years, including cardiac death, PCI, MI, revascularization, and total major adverse cardiac events. There was no difference in clinical outcomes up to 5 years between the two groups (
Table 5). We also investigated the number of patients who underwent repeat CAG due to recurrent chest pain, and the results thereof are shown in
Table 5. We did not report the percentage of patients who complained of recurrent chest pain.
Table 5
Cardiovascular Outcome and Recurrent Angina Up to 5 Years
|
Variables, % |
Entire patients |
Matched patients |
|
Diltiazem (n=842) |
Dual (n=1899) |
p value |
Diltiazem (n=811) |
Dual (n=811) |
p value |
|
Total death |
0.0 |
0.9 |
0.050 |
0.0 |
1.1 |
0.039 |
|
Cardiac death |
0.0 |
0.3 |
0.156 |
0.0 |
0.1 |
1.000 |
|
Myocardial infarction |
1.0 |
0.6 |
0.564 |
1.0 |
0.2 |
0.492 |
|
Revascularization |
0.6 |
1.4 |
0.264 |
0.6 |
1.1 |
0.811 |
|
Total major adverse cardiac events |
0.8 |
2.3 |
0.052 |
0.6 |
2.2 |
0.248 |
|
Recurrent angina |
8.3 |
0011.7 |
0.001 |
8.3 |
8.2 |
0.276 |

We analyzed the clinical outcomes up to 5 years between the two groups, and the results were shown as Kaplan-Meier curves (
Figs. 1 and
2). After 5 years, the diltiazem group and the combined treatment group showed similar cumulative incidences of MACE and recurrent chest pain requiring repeat CAG.
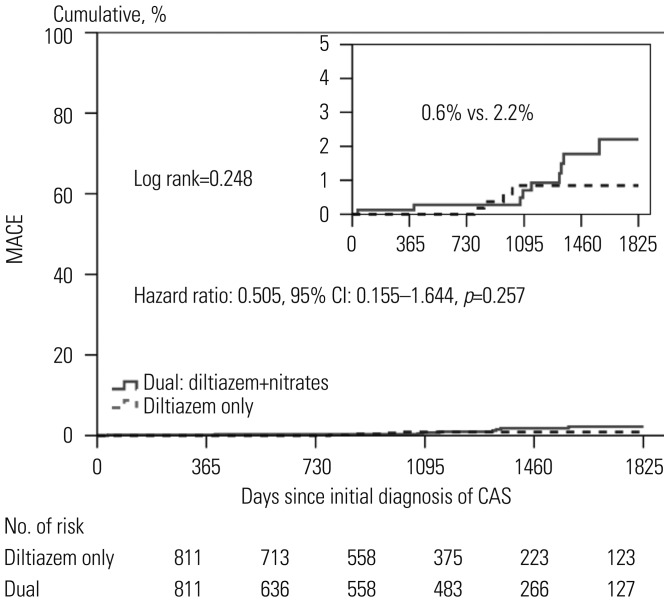 | Fig. 1Kaplan-Meier curves of major adverse cardiac events (MACE) between the two groups. Figure shows the cumulative incidences of MACE includings composite of death, myocardial infarction, or de novo percutaneous coronary intervention MACE. The diltiazem only group (indicated by dotted line) received diltiazem without nitrates. The dual group (indicated by black) received both of diltiazem and nitrates. CAS, coronary artery spasm.
|
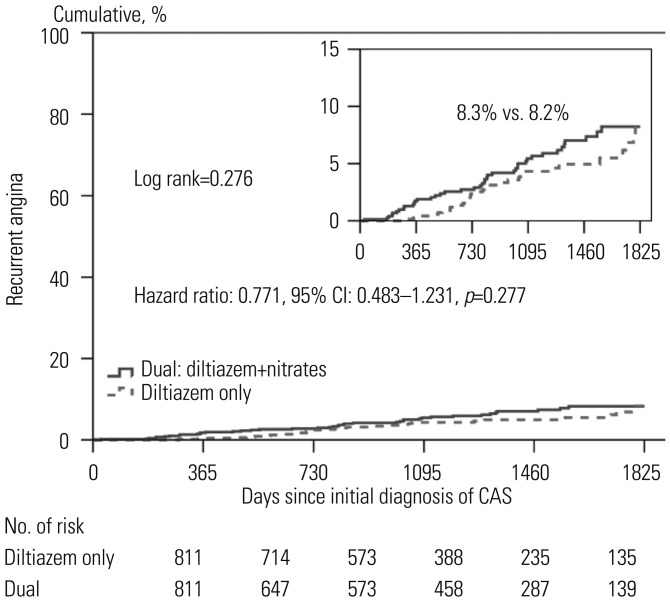 | Fig. 2Kaplan-Meier curves of recurrent angina requiring coronary angiography between the two groups. Figure shows the cumulative incidences of recurrent angina requiring repeat coronary angiography. The diltiazem only group (indicated by dotted line) received diltiazem without nitrates. The dual group (indicated by black) received both of diltiazem and nitrates. CAS, coronary artery spasm.
|
We evaluated independent predictors associated with MACEs and recurrent angina requiring repeat CAG by adjusting for possible confounding factors, including diltiazem only regimen; age; male gender; diabetes mellitus; hypertension; dyslipidemia; diabetes mellitus; current smoking; and current medications including aspirin, beta blockers, trimetazidine, diuretics, angiotensin receptor blockers, angiotensin converting enzyme inhibitors, statins, and myocardial bridge. The results are listed in the
Table 5 and
Figs. 1 and
2. Multivariate analysis showed that beta blocker therapy [odds ratio (OR)=7.585,
p<0.001] was an independent predictor of MACE (
Table 5,
Fig. 1), and male gender (OR=1.834,
p=0.026) was an independent predictor for recurrent angina requiring repeat CAG (
Fig. 2). The diltiazem alone regimen was not an independent protector for MACE and recurrent angina.
Go to :

DISCUSSION
The main finding of the present study is that there is no significant difference in clinical outcomes, including MACE and recurrent angina requiring repeat CAG, in patients with significant CAS when treated with the diltiazem alone, compared to combined treatment with diltiazem and nitrate, up to 5 years. In the present study, we matched patients using PSM in relation to demographics (e.g., age, gender, BMI) clinical risk factors (e.g., blood pressure, heart rate, etc., lipid levels, diabetes), other medications (e.g., beta-blockers, statins); they differed only by presence or absence of chronic nitrate treatment as part of their medications. In conclusion, the long-term nitrate therapy added to diltiazem in these patients produced no long-term additional beneficial effect over diltiazem alone.
The pathophysiological basis of nitrate therapy stems from the observations noted in multiple previous studies that support the notion of NO deficiency in CAS. NO is an endothelial derived vasodilator that is specifically blocked by L-monomethyl-arginine (L-NMMA) causing vasoconstriction.
67 Nitrates work by an endothelium independent pathway and cause vasodilation by conversion to NO. The response to nitrates has been found to have inverse correlation to L-NMMA. The smaller the vasoconstrictive effect of L-NMMA, the larger the vasodilatory effect of nitrates, suggesting deficiency of endothelial derived NO in CAS.
8 NO also suppresses endothelin I and angiotensin II, which are potent vasoconstrictors and proliferators of vascular smooth muscle.
910 Several genetic polymorphisms involving endothelial-nitric oxide synthase gene have been shown to be significantly associated with CAS.
11
Even though it is believed that endothelial dysfunction related to decrease NO activity causes CAS with vasospastic angina, a regimen of a nitrate added to a CCB could not improve clinical outcomes.
12 There is consistent evidence to suggest that in patients with variant angina, a hyper reactivity of VSMC contraction of the coronary artery wall is responsible for CAS, and an impairment of endothelium-mediated vasodilation seems unlikely to be responsible by itself for CAS, although endothelial dysfunction might induce CAS by vasoconstrictors at the site of predisposed segments.
13 Therefore, it can be interpreted that a CCB affecting vasoconstriction by reaction of VSMC should be more important for CAS, as compared with nitrate affecting endothelial dysfunction.
There is a similar study regarding long-term prognosis of chronic nitrate therapy combined to CCB for CAS-related angina. Therein, chronic nitrate therapy did not improve long-term prognoses, including MACE, when combined with CCBs. Furthermore, the CAS patients with multiple nitrates would have increased risk for cardiac events.
14 This study was conducted in a small number of patients, compared to our study population, although they showed consistent results with our study. However, no other study has compared CCB alone therapy and combined therapy including nitrate and CCB in relation to long-term clinical outcomes, which is the strength of this study.
The management of chronic vasospastic angina suggests the use of high dose CCB (verapamil or diltiazem or nifedipine) and long acting isosorbide dinitrate or mono-nitrate. Dihydropyrindine and non-dihydropyridine CCB represent the main therapy because they reduce, prevent, and resolve spasm attacks and thus angina and arrhythmias. In this study, many patients complained of spontaneous vasospastic episodes with pounding feelings. Therefore, we preferentially used diltiazem for the treatment of coronary spastic angina. However, for patients with elevated blood pressure, we preferentially used nifedipine for the treatment of coronary spastic angina.
Racial differences in the incidence of CAS between East Asian countries (Japan 40.9 to 79%) and Caucasians have been reported.
15 A previous study in Western countries showed different incidences in CAS, among German, French, and Japanese individuals. In a white German population with acute coronary syndrome without a culprit lesion, 49% had a positive Ach test, whereas 16% of French patients did. In the present study, 54.7% of patients who presented with anginal chest pain were diagnosed with CAS by Ach provocation test. The marked reduction in cigarette smoking and the wide-spread use of calcium antagonist therapy could affect the lower incidence of CAS in Caucasian populations. Although the CAS incidence of Caucasian appears to be relatively lower than Asian, it will be valuable to design similar studies in Western populations to establish optimal antianginal therapy regimens.
In our study, male sex, statins, and beta blockers were independent predictors for major cardiac events and repeat CAG. Males had a higher prevalence of coronary risk factors, including smoking, and may be associated with higher incidence of clinical events in general. In our study population, patients who used statin likely had more advanced atherosclerotic cardiovascular disease and dyslipidemia. Thus, statin was not a protective predictor for clinical events in this clinical setting. Non-selective beta blockers (like propranolol) convert the effects of sympathetic stimulation in to a pure alpha adrenergic vasoconstrictor response, thus they can exacerbate and prolong vasospasm.
16 However, since CAS can occur in patients with stable angina as well, concomitant use of calcium channel and selective beta blockers can be considered in CAS, although it should be noted that this can exacerbate spasm recurrence.
In this study, there were several limitations. First, the present study was an observational study and was retrospective in design. Because of the design of study, the cause-result relationship was not established. Second, in this study, the incidences of conductive rhythm disorder were not analyzed. When we designed the registry, we did not consider it because the clinical course of CAS was considered to be relatively benign, compared to that of patients with significant fixed coronary artery stenosis. Third, East Asian countries, including Korea and Japan, have reported higher incidences of CAS than Western countries by Ach provocation test. Since this registry consists of Korean patients alone, the present results may not be extended to all patients worldwide.
17 Fourth, recently, several studies adopted the definition of significant CAS regarding more than 75% narrowing documented by Ach provocation test.
181920 According to guidelines for diagnosis and treatment of patients with vasospastic angina, CAS is defined as transient, total, or sub-total occlusion (>90% stenosis) of a coronary artery with signs/symptoms of myocardial ischemia (angina pain and ischemic ST changes).
21 However, we modified the positive Ach provocation test criteria for patient safety and efficacy, as published elsewhere; and in the present study, significant CAS was defined as focal or diffuse severe transient luminal narrowing (>70%) with or without chest pain or ischemic ECG change, such as ST-T segment elevation, depression (≥1 mm) or T wave inversion.
451722 Ach provocation test was performed only on the left coronary artery for patient safety. Most of the Ach provocation tests were performed in an outpatient setting and undertaken with 4F transradial route. Ach provocation into the right coronary artery frequently causes significant AV nodal block, which needs temporary pacemaker support and ventricular tachycardia/ventricular fibrillation can develop. Fifth, the present study only addressed epicardial CAS by the effect of the Ach provocation test. Therefore, intracoronary Ach provocation test-induced limitations in microcirculation were not assessed. This could affect the consequence of the prognosis. Fifth, the long-term use of medications was not strictly controlled by the researchers, because this study was performed as a retrospective and observational design. Therefore, the follow-up period varied in individual patients; the definite 'duration' of maintenance in medication varied according to their physicians' discretion; and the relationship between compliance or changes in medications during the follow up period and patients outcome was not evaluated. This is a practical study limitation. However, when we consider our clinical practice, once symptoms are stabilized, physicians tend to maintain their medication at least for six months due to safety issues. In the future, a randomized controlled trial would be needed to arrive at a final conclusion. Sixth, management decisions were made according to the discretion of each attending physician, and we were unable to check whether the use of CCB plus nitrate was related to the more severe angina symptoms or sign. However, when we consider routine clinical practice, combination therapy tends to garner greater usage with more severe symptoms. Seventh, statins were not a protective factor for the incidence of recurrent angina and MACE, and the statin use paradox may be related to the following two reasons: 1) the patients of this study were a low risk population with minimal fixed stenosis or normal looking coronary arteries, thus they do not represent the entire population of cardiovascular disease who might see greater benefit with chronic statin therapy; and 2) the rate of use of statin was relatively low (36.9% vs. 38.2%,
p value 0.608), thus the impact of chronic statin therapy might be limited to show the difference in clinical outcomes. We believe a well-designed randomized study with larger study population would be needed to arrive at a final conclusion on the statin use paradox.
In this study, we performed a provocation test in patients with spontaneous vasospastic episode. Definite vasospastic angina is defined as the event of an ischemic ECG change during a spontaneous vasospastic episode, and the provocation test is not required. However, if the ischemic ECG changes are equivocal, then the provocation test is required, and a positive test would be indicative of definite vasospastic angina, whereas a negative test warrants a diagnosis of suspected vasospastic angina.
21 Therefore, we performed the provocation test in patients with spontaneous vasospastic episode with ischemic ECG changes are equivocal and vasoconstriction of more than 70% in diameter by invasive CAG are diagnosed as significant CAS, not definite CAS.
Clinically there is a common belief that addition of nitrate to CCB would be better to control more symptomatic CAS, and it has been a common regimen for patients with recurrent anginal symptoms with CCB alone. However, in this study, antianginal medical therapy with diltiazem alone did not differ with a combined regiment with diltiazem and nitrate in reducing not only the incidence of recurrent angina, but also the incidence of adverse clinical events up to 5 years. As discussed above, this result suggests that VSMC is more directly related to the pathophysiology of CAS, compared to endothelial dysfunction of deficient bioactivity of NO. Considering the side effect of nitrates, which include headaches, dizziness, and facial flush, treating CAS patients with CCB alone may be warranted in terms of the clinical outcomes presented in this study.
In conclusion, despite expected improvement in endothelial function and relieving vasoconstriction, combination of diltiazem and nitrate treatment were not superior to diltiazem alone in reducing MACE and ischemic symptoms for up to 5 years.
Go to :












 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download