Abstract
Purpose
Rearrangement of the proto-oncogene rearranged during transfection (RET) has been newly identified potential driver mutation in lung adenocarcinoma. Clinically available tyrosine kinase inhibitors (TKIs) target RET kinase activity, which suggests that patients with RET fusion genes may be treatable with a kinase inhibitor. Nevertheless, the mechanisms of resistance to these agents remain largely unknown. Thus, the present study aimed to determine whether epidermal growth factor (EGF) and hepatocyte growth factor (HGF) trigger RET inhibitor resistance in LC-2/ad cells with CCDC6-RET fusion genes.
Materials and Methods
The effects of EGF and HGF on the susceptibility of a CCDC6-RET lung cancer cell line to RET inhibitors (sunitinib, E7080, vandetanib, and sorafenib) were examined.
Results
CCDC6-RET lung cancer cells were highly sensitive to RET inhibitors. EGF activated epidermal growth factor receptor (EGFR) and triggered resistance to sunitinib, E7080, vandetanib, and sorafenib by transducing bypass survival signaling through ERK and AKT. Reversible EGFR-TKI (gefitinib) resensitized cancer cells to RET inhibitors, even in the presence of EGF. Endothelial cells, which are known to produce EGF, decreased the sensitivity of CCDC6-RET lung cancer cells to RET inhibitors, an effect that was inhibited by EGFR small interfering RNA (siRNA), anti-EGFR antibody (cetuximab), and EGFR-TKI (Iressa). HGF had relatively little effect on the sensitivity to RET inhibitors.
The identification of oncogenic drivers in tumor cells, coupled with the targeting of these proteins by small molecule inhibitors, has emerged as an increasingly successful treatment strategy for non-small cell lung cancer (NSCLC). NSCLC with epidermal growth factor receptor (EGFR) gene mutation is commonly sensitive to the EGFR inhibitors gefitinib and erlotinib. NSCLC with anaplastic lymphoma kinase (ALK)-fusion is sensitive to the ALK inhibitor crizotinib. Lung cancer with ROS1 (ROS1 proto-oncogene receptor tyrosine kinase) fusion also responds to crizotinib.123
Rearranged during transfection (RET) is a targetable driver mutation under investigation in NSCLC. RET is a receptor tyrosine kinase (RTK) involved in cell proliferation, neuronal navigation, cell migration, and cell differentiation.4 The incidence of RET fusions (KIF5B-RET or CCDC6-RET) is approximately 1% in patients with primary lung tumors.5 The oncogenic potential of RET fusion has been demonstrated in vitro in transfected NIH3T3 and Ba/F3 cells. RET inhibition with vandetanib, sunitinib, or sorafenib results in loss of cell viability and abrogation of the transformed phenotype. This result suggests that RET could be a druggable target.678 Drilon, et al.9 reported partial responses in two cases of RET fusion-positive NSCLC during phase 2 trials with the RET inhibitor cabozantinib. This result provides clinical validation of RET fusions as an oncogenic alteration in lung cancers.
Meanwhile, however, about 30% of all NSCLC patients with genetic driver alterations show intrinsic resistance to small molecule inhibitors.101112 In addition, almost all patients with oncogenic drivers who respond to small molecules ultimately develop resistance to these agents. Therefore, understanding the mechanism of resistance to targeted therapy is essential.
The tumor microenvironment is gaining acceptance as an essential factor of therapeutic responses. For instance, autocrine, paracrine, and endocrine activation of oncogenic receptor kinases can disrupt therapeutic inhibition by sustaining activation of common intracellular signaling pathways.13 Wilson, et al.14 reported that EGF, hepatocyte growth factor (HGF), fibroblast growth factor (FGF), and neuregulin confer drug resistance on tumor-derived cell lines that have oncogenic RTK signaling. The EGFR family of receptors is of particular interest in lung cancer.15 Many lung cancer cells express EGFR and MET. These cells, along with others in their microenvironment, also express ligands of EGFR and MET, suggesting that these receptors and ligands control the sensitivity of cancer cells to small molecule inhibitors in their microenvironment.1416 Nevertheless, the role of the microenvironment in the sensitivity of RET-fusion-positive lung cancer cells to RET-tyrosine kinase inhibitors (TKIs) has not been determined.
In the present study, we aimed to examine whether EGF and HGF in the microenvironment of RET fusion-positive lung cancer cells triggers resistance to RET inhibitors, including E7080, a multi-targeted TKI that inhibits RET, vascular endothelial growth factor receptor (VEGFR)-2, VEGFR-1, FGFR-1, and platelet-derived growth factor RTKs.
We selected a human lung adenocarcinoma cell line, LC-2/ad, that has a CCDC6-RET fusion.17 We identified the CCDC6-RET fusion in LC-2/ad by fusion-specific reverse transcription-polymerase chain reaction (RT-PCR) (data not shown). The LC-2/ad cell line was obtained from the RIKEN cell bank (Japan). Human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Culture Collection. LC-2/ad cells were cultured in a 1:1 mixture of RPMI1640/Ham’s F-12 medium (Gibco, Carlsbad, CA, USA), supplemented with 25 mM HEPES, 15% fetal bovine serum, penicillin (100 U/mL), and streptomycin (50 µg/mL), in a humidified CO2 incubator at 37℃. HUVEC cells were maintained in endothelial cell basal medium-2 and growth supplements (Lonza, Anaheim, CA, USA), and passages 2 through 5 were used for in vitro assays.
E7080, sorafenib, vandetanib, and TAE-684 were obtained from Seleck Chemicals. Sunitinib malate was purchased from Sigma-Aldrich (St. Louis, MO, USA). The anti-human EGFR antibody cetuximab was obtained from Merck Serono. Recombinant EGF and HGF were from R&D Systems (Minneapolis, MN, USA).
LC-2/ad cells were seeded in 96-well tissue culture plates at 5000 cells per well. Cells were cultured in RPMI1640 with 5% fetal bovine serum and incubated for 24 hours. EGF and HGF were added for incubation for 2 hours. Sunitinib, E7080, vandetanib, or sorafenib were added to each well and incubation was continued for an additional 72 hours. For analysis, plates were incubated for 30 minutes at room temperature. Each well received 100 µL of CellTiter-Glo Luminescent Cell Viability assay buffer (Promega, Madison, WI, USA), and plates were shaken for 10 minutes. Luminescence was quantified with L Max II 384 (Molecular Devices, Sunnyvale, CA, USA). Percent growth was calculated relative to untreated controls. Assays were carried out at least in triplicate with results based on three independent experiments. The consequences of ligand exposure on drug response were categorized as: 1) no rescue, in which addition of ligand did not affect the drug response; 2) partial rescue, in which ligand partially abrogated the treatment response; or 3) complete rescue, in which ligand right-shifted the IC50 curve >10-fold or completely suppressed the drug response.14
LC-2/ad cells were seeded in six-well plates and incubated overnight. Growth medium was removed from plates before transfection. After adding 500 µL of opti-MEM (Gibco), cells were transfected with EGFR-specific small interfering RNA (siRNA) (100 pmol) or negative control siRNA using 3.5 µL of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. After 6 hours, cells were washed with PBS and incubated with growth media for 24 hours. Transfected cells were co-cultured with HUVEC cells.
Cells were co-cultured in Transwell collagen-coated chambers separated by 8-µm (Corning, Lowell, MA, USA) pore-size filters. LC-2/ad cells or EGFR-specific siRNA-transfected LC-2/ad cells were seeded in 24-well plates at 8000 cells per well and incubated with or without cetuximab (2 µg/mL) or gefitinib (1 µM). After 24 hours, cells were co-cultured with or without HUVEC cells (10000 cells/300 µL) in the upper chamber. After 24 hours, LC-2/ad cells were incubated with RET inhibitors (sunitinib, E7080, vandetanib, or sorafenib) for 72 hours. After upper chamber removal, wells received 100 µL of CellTiter-Glo Luminescent Cell Viability assay buffer (Promega), and plates were shaken for 10 minutes. Luminescence was quantified, and percent growth was assessed as described above. Assays were carried out at least in triplicate, with results based on three independent experiments.
Samples were denatured in buffer containing 60 mM Tris/pH 6.8, 25% glycerol, 2% sodium dodecyl sulfate (SDS), and 14.4 mM 2-mercaptoethanol with 0.1% bromophenol blue, and boiled for 5 minutes. SDS polyacrylamide gels (Bio-Rad, Hercules, CA, USA) were loaded with 25 µg of total protein per lane. Prestained molecular weight markers (Bio-Rad) were used as standards. Electrophoresed samples were transferred to polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ, USA). After transfer, membranes were blocked with 3% bovine serum albumin in Tris-phosphate-buffered saline (TPBS; 200 mM Tris/pH 7.0, 1.37 M NaCl, 1% Tween-20) for 1 hour at room temperature. Membranes were then incubated with anti-RET, anti-phospho-RET, anti-EGFR, anti-phospho-EGFR, anti-AKT, anti-phospho-AKT, anti-ERK, anti-phospho-ERK, or anti-beta actin (1:1000; Cell Signaling Technology, Danvers, MA, USA) overnight at 4℃, washed as before, and incubated with goat anti-rabbit peroxidase-conjugated secondary antibody (1:4000; Cell Signaling Technology) for 2 hours at room temperature. Membranes were then washed, and expressed proteins were detected with Pierce ECL plus western blotting substrate (Pierce, Rockford, IL, USA). Experiments were conducted at least three times independently.
We examined the sensitivity of the human LC-2/ad lung cancer cell line, containing CCDC6-RET translocations, to RET inhibitors sunitinib, E7080, vandetanib, sorafenib, and TKIs. Human LC-2/ad cells were insensitive to the EGFR-TKIs gefitinib (a reversible EGFR-TKI) and TAE-684 (selective for ALK), but were sensitive to the RET-TKIs sunitinib, E7080, vandetanib, and sorafenib (Fig. 1). IC50 values were 0.66 µM for sunitinib, 0.77 µM for E7080, 0.22 µM for vandetanib, and 1.1 µM for sorafenib.
Since EGF and HGF are associated with drug resistance in lung cancer,14 we explored the effect of the EGFR ligand EGF and the MET ligand HGF on the sensitivity of RET-fusion lung cancer cells to RET inhibitors. In the absence of RET inhibitors, EGF and HGF slightly increased the viability of LC-2/ad cells. EGF dose-dependently reduced the sensitivity to RET inhibitors (Figs. 1 and 2). EGF was a broadly active ligand for sensitivity to sunitinib, E7080, vandetanib, and sorafenib. HGF had relatively little effect on sensitivity to RET inhibitors (Table 1).
To assess the mechanism by which EGF reduced cell sensitivity to RET inhibitors, we analyzed the phosphorylation status of RET, EGFR, and their downstream molecules in LC-2/ad cells using western blots. RET inhibitors sunitinib, E7080, vandetanib, and sorafenib inhibited RET phosphorylation, suppressing phosphorylation of AKT and ERK (Fig. 3, lane 1 and 2). EGF stimulated phosphorylation of EGFR in LC-2/ad cells (Fig. 3, lane 3). In the presence of EGF, vandetanib and sorafenib, but not sunitinib and E7080, decreased EGFR phosphorylation (Fig. 3, lane 4, rows 3 and 4). No RET inhibitors diminished phosphorylation of AKT and ERK with cotreatment of EGF and RET inhibitors, compared to inhibitor only (Fig. 3, lanes 2 and 4, rows 5–8). This result suggested that despite the presence of RET inhibitors, EGF promoted resistance by reactivating AKT and ERK signaling.
To establish novel strategies for treating EGF-triggered resistance to RET inhibitors, we examined the effect of combinations of RET inhibitors and EGFR inhibitors. Combined treatment with gefitinib, a reversible EGFR-TKI, resensitized LC-2/ad cells to RET inhibitors even in the presence of EGF (Fig. 4). Western blots showed that gefitinib inhibited EGF-induced EGFR phosphorylation. The combination of RET inhibitors and gefitinib inhibited phosphorylation of AKT and ERK by RET and EGFR even in the presence of EGF (Fig. 3, lanes 4 and 7, rows 5–8).
HUVEC cells are reported to produce discernible levels of EGF.18 Co-culture of LC-2/ad cells with endothelial cells reduced sensitivity to RET inhibitors. To confirm the involvement of EGFR in the resistance of RET inhibitors induced by EGF produced by HUVECs, we knocked down EGFR using specific siRNAs and downregulated the activation in LC-2/ad cells using cetuximab and gefitinib (Fig. 5). RET inhibitors inhibited cell viability, and co-culture with HUVECs induced resistance in cells treated with scrambled siRNA. In EGFR siRNA-treated cells, resistance to RET inhibitors was not induced by co-culture with HUVEC. These results indicated that HUVEC-triggered resistance to RET inhibitors is mediated by EGFR. In addition, resistance induced by co-culture of LC-2/ad cells was inhibited by anti-EGFR antibody and a reversible EGFR-TKI (Figs. 6 and 7). These results suggested that host stromal cells such as endothelial cells might regulate sensitivity to RET inhibitors by secreting EGF.
Our results demonstrated that the RET-translocated LC-2/ad NSCLC cell line is sensitive to RET inhibitors, which inhibited RET and its downstream targets. We demonstrated that endothelial cells, which are components of the tumor microenvironment, confer resistance to RET inhibitors sunitinib, E7080, vandetanib, and sorafenib by activating bypass survival signals of EGFR.
Over the past few years, kinase fusions in NSCLC have emerged as targetable driver events. The antitumor effect of crizotinib on NSCLC with ALK and ROS1 fusions calls attention to how the availability of multikinase inhibitors has expedited this effort. In spite of this progress, the time between the discovery of genetic driver alterations, the demonstration of their activity, and final approval of a corresponding targeted agent remains lengthy and is typically measured in years. However, a prospective trial of the RET inhibitor cabozantinib was initiated only a few months after discovery of RET fusions.9 A preliminary report for the first three patients treated with cabozantinib with RET fusion-positive NSCLC confirmed partial responses in two. The third patient had prolonged stable disease approaching 8 months. All three patients remain progression-free on treatment. This report provided early clinical validation of RET fusions as drivers in NSCLC, and proposed that RET inhibition may represent a new therapeutic paradigm for RET fusion lung cancer. However, one-third (33%) of the patients did not exhibit a clinical response; this result suggests that innate mechanisms render a substantial proportion of tumor cells resistant to RET inhibitor, similar to other oncogenic cancer cells treated with matched TKIs. We showed here that endothelial cells, a component of the tumor microenvironment, cause a heterogeneous response to RET inhibitors by activating bypass survival signals of EGFR with the EGFR ligand EGF. EGF-triggered resistance was prevented by gefitinib and cetuximab; these drugs are approved for treatment of NSCLC. In addition, the ligand reactivated at least one of the downstream survival signaling PI3K-AKT and ERK/MAPK pathways commonly engaged by RTKs despite the presence of RET inhibitors.
Our preclinical results on RET inhibitors are in line with other recently published studies that have identified activation of the EGFR axis as a possible mechanism for bypassing the inhibition of ALK phosphorylation conferred by crizotinib and more potent ALK TKIs. Furthermore, upregulation of EGFR ligands and EGFR is consistently observed.31219 Our discovery of EGF-mediated resistance to RET inhibitors should be distinguished from recent reports that suggest dysregulation of second-site mutations at codon 790 of EGFR, MET amplification for EGFR-TKIs, or ALK-resistance mutations or amplification as mechanisms of resistance to specific TKIs.2021 These reports studied the emergence of late drug resistance (i.e., following exposure to a drug for many months). We suggested that EGF-secreting stromal cells could confer immediate resistance to RET inhibitors. Whether EGFR pathway is involved in acquired resistance remains to be determined.
Although the role of EGFR-TKIs in the treatment of patients with wild-type EGFR is still contentious, selected patients with targetable mutations (e.g., RET, ALK) seem to benefit from EGFR-TKI therapy combined with corresponding target drugs. Further clinical trials are warranted to evaluate the efficacy and feasibility of using a combination of EGFR inhibitors to overcome RET-inhibitor resistance. For ALK-fusion NSCLC, ongoing clinical trials of crizotinib plus erlotinib and crizotinib plus dacomitinib have been initiated.
We discovered that resistance was partially induced to vandetanib, an inhibitor of EGFR, and sorafenib, an inhibitor of Raf kinases. Resistance to sunitinib and E7080 were complete. Our findings suggested that EGF-triggered resistance may be more profound against sunitinib and E7080. Future clinical trials may reveal the class of RET inhibitors that are most beneficial for RET-fusion NSCLC. Whether the ligand-triggered resistance is an independent mechanism or provides partial resistance when combined with another mechanism remains an open question. Analyses of serial biopsy specimens for EGFR activation are anticipated from clinical trials in NSCLC patients with RET fusion.
In a study of lung adenocarcinoma cell lines, we found that E7080 inhibits growth of CCDC6-RET-positive LC-2/ad cells but not A549 (KRAS-mutation positive), NCI-H3122 (ALK-fusion positive), or PC-9 (EGFR-mutation positive) cells (data not shown). E7080 inhibits VEGFR-2 and suppresses angiogenesis in human xenograft models.22 The anti-angiogenic activity of E7080 was confirmed in a phase I clinical study using circulating endothelial cells as a biomarker.23 The antitumor activity of E7080 against lung adenocarcinoma cells positive for RET-gene fusion demonstrated in this study were distinct from the previously observed anti-angiogenic activity of E7080 based on several observations.2223
We investigated the role of HGF and EGF in resistance of LC-2/ad cells. HGF reduces the sensitivity of EGFR-mutant or ALK-fusion lung cancer cells by activating MET receptors.2024 Resistance to TAE-684, a selective ALK inhibitor, was induced by both EGFR ligands and HGF. However, our data showed that, for RET inhibitors, HGF had relatively little effect on resistance to TKIs. Further investigation is needed to determine why EGFR is a potent bypass track in these RET-fusion cells, while HGF is not.
In conclusion, we found that EGF causes resistance to RET inhibitors sunitinib, E7080, vandetanib, and sorafenib by activating bypass survival signals. In addition, co-culture with endothelial cells conferred resistance to RET inhibitors by EGF. This observation suggested that paracrine receptor activation by the microenvironment may be an important mechanism for inducing innate resistance to molecular-targeted drugs in oncogene-activated lung cancer cells. If EGFR activation is confirmed as a predominant mechanism of TKI-induced resistance in RET-fusion patient-derived tumors, combination of RET and EGFR TKIs may be beneficial to overcome resistance. The data presented in this report suggest that the antitumor activities of E7080 against RET gene fusion lung cancer cells are conferred by RET inhibition.
Figures and Tables
Fig. 1
EGFR ligand reduced the sensitivity of LC-2/ad cells to RET inhibitors in vitro. CCDC6-RET lung cancer cells were sensitive to RET inhibitors sunitinib, E7080, vandetanib, and sorafenib. LC-2/ad cells were pretreated with or without EGF (100 ng/mL) or HGF (50 ng/mL) for 24 hours and incubated with several concentrations of RET inhibitors sunitinib, E7080, vandetanib, or sorafenib. Cell growth was measured after 72 hours by celltiter-glo luminescent cell viability assay kits (Promega). Data are the mean of three independent experiments, each in triplicate. Bars, standard deviation. HGF, hepatocyte growth factor; EGFR, epidermal growth factor receptor; RET, rearranged during transfection.

Fig. 2
Dose-dependent effects of EGF. LC-2/ad cells were incubated with several concentrations of EGF for 24 hours. LC-2/ad cells were incubated with RET inhibitors sunitinib (0.66 µM), E7080 (0.77 µM), vandetanib (0.22 µM) or sorafenib (1.1 µM). Cell growth was determined after 72 hours by celltiter-glo luminescent cell viability assay kit (Promega). Data are the mean of three independent experiments, each in triplicate. Bars, standard deviation. EGF, epidermal growth factor; RET, rearranged during transfection.

Fig. 3
LC-2/ad, CCDC6-RET lung cancer cells were highly sensitive to RET inhibitors. Sunitinib (A), E7080 (B), Vandetanib (C), Sorafenib (D). EGF receptor (EGFR) ligand, EGF, or activated EGFR triggered resistance to RET inhibitors by transducing bypass survival signaling through ERK and AKT. LC-2/ad cells were treated with or without gefitinib (1 µM) and/or EGF (100 ng/mL) for 2 hours with or without RET inhibitor at half-maximum inhibitory concentration (IC50) for 1 hour. Cells were lysed and indicated proteins were detected by western blots. Shown are representative of three independent experiments. EGFR, epidermal growth factor receptor; RET, rearranged during transfection.
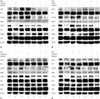
Fig. 4
Abrogation of EGF-triggered resistance to RET inhibitors by EGFR-TKI. Sunitinib (A), E7080 (B), Vandetanib (C), Sorafenib (D). LC-2/ad cells were incubated with or without gefitinib (1 µM) for 2 hours and/or EGF (100 ng/mL) for 24 hours with or without RET inhibitors at half-maximum inhibitory concentration (IC50), with cell growth determined after 72 hours. Cell growth was measured by celltiter-glo luminescent cell viability assay kit (Promega). *p<0.05 (one-way ANOVA). Data are the mean of three independent experiments, each in triplicate. Bars, standard deviation. EGFR, epidermal growth factor receptor; RET, rearranged during transfection; TKI, tyrosine kinase inhibitor.

Fig. 5
Reduction of RET-fusion lung cancer cell sensitivity to RET inhibitors by co-culture with HUVEC endothelial cells. EGFR-specific siRNAs were transfected into LC-2/ad cells. After 24 hours, cells were cultured with or without HUVEC cells. After 24 hours, cells were incubated with RET inhibitors sunitinib, E7080, vandetanib, or sorafenib) at half-maximum inhibitory concentration (IC50) for kinase inhibitors for 72 hours. Cell growth was determined by celltiter-glo luminescent cell viability assay kit (Promega). *p<0.05 (one-way ANOVA). Data are the mean of three independent experiments, each in triplicate. Bars, standard deviation. EGFR, epidermal growth factor receptor; RET, rearranged during transfection; HUVEC, human umbilical vein endothelial cell; siRNA, small interfering RNA.

Fig. 6
Reduction of RET-fusion lung cancer cell sensitivity to RET inhibitors by co-culture with HUVEC endothelial cells. LC-2/ad cells were incubated with or without Cetuximab (2 µg/mL). After 24 hours, cells were cultured with or without HUVEC cells. After 24 hours, cells were incubated with RET inhibitors for 72 hours. Cell growth was determined as above. *p<0.05 (one-way ANOVA). Data are the mean of three independent experiments, each in triplicate. Bars, standard deviation. HUVEC, human umbilical vein endothelial cell; RET, rearranged during transfection.

Fig. 7
Reduction of RET-fusion lung cancer cell sensitivity to RET inhibitors by co-culture with HUVEC endothelial cells. LC-2/ad cells were incubated with or without gefitinib (1 µM). After 24 hours, cells were cultured with or without HUVEC cells and incubated with RET inhibitors as above. Cell growth was determined as above. *p<0.05 (one-way ANOVA). Data are the mean of three independent experiments, each in triplicate. Bars, standard deviation. HUVEC, human umbilical vein endothelial cell; RET, rearranged during transfection.

ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2012866).
Hyun Chang received a research grant from Astrazenaca. No potential conflicts of interest were disclosed by the other authors.
References
1. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139.

2. Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012; 30:863–870.
3. Tanizaki J, Okamoto I, Okabe T, Sakai K, Tanaka K, Hayashi H, et al. Activation of HER family signaling as a mechanism of acquired resistance to ALK inhibitors in EML4-ALK-positive non-small cell lung cancer. Clin Cancer Res. 2012; 18:6219–6226.

4. Eng C. RET proto-oncogene in the development of human cancer. J Clin Oncol. 1999; 17:380–393.
5. Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012; 30:4352–4359.

6. Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012; 18:375–377.

7. Lipson D, Capelletti M, Yelensky R, Otto G, Parker A, Jarosz M, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012; 18:382–384.

8. Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012; 18:378–381.

9. Drilon A, Wang L, Hasanovic A, Suehara Y, Lipson D, Stephens P, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 2013; 3:630–635.

10. Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W, et al. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011; 17:5530–5537.

11. Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010; 363:1734–1739.

12. Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012; 4:120ra17.

14. Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012; 487:505–509.

15. Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer--is it becoming a reality? Nat Rev Clin Oncol. 2010; 7:401–414.

16. Meert AP, Martin B, Delmotte P, Berghmans T, Lafitte JJ, Mascaux C, et al. The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J. 2002; 20:975–981.

17. Matsubara D, Kanai Y, Ishikawa S, Ohara S, Yoshimoto T, Sakatani T, et al. Identification of CCDC6-RET fusion in the human lung adenocarcinoma cell line, LC-2/ad. J Thorac Oncol. 2012; 7:1872–1876.

18. Yamada T, Takeuchi S, Nakade J, Kita K, Nakagawa T, Nanjo S, et al. Paracrine receptor activation by microenvironment triggers bypass survival signals and ALK inhibitor resistance in EML4-ALK lung cancer cells. Clin Cancer Res. 2012; 18:3592–3602.

19. Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011; 71:6051–6060.

20. Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013; 31:1105–1111.
21. Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012; 109:E2127–E2133.
22. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M, et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008; 14:5459–5465.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download