INTRODUCTION
The inferior vena cava (IVC) is a rare site of focal atrial tachycardia (AT), and AT can occur long time after open heart surgery.1 Although three-dimensional (3D)-electroanatomical mapping provides detailed electrophysiology, a review of surgical record is important to understand the mechanism of tachycardia.2 Here, we report a case of a successfully ablated focal AT originating from the IVC-right atrial (RA) junction, confirmed by 3D electroanatomical mapping 17 years after surgery for atrial septal defect (ASD).
CASE REPORT
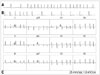
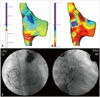
A 20-year-old woman was admitted to our hospital because of recurrent episodes of palpitation. She had a history of ASD patch closure surgery at the age of 3 years and permanent pacemaker implantation (DDDR type) for sick sinus syndrome at the age of 13 years. For ASD closure, the surgeon utilized an autologous pericardial patch and performed venous cannulations at the superior vena cava (28 Fr) and the IVC (30 Fr) for cardiopulmonary bypass. Computed tomography and transthoracic echocardiography revealed normal left ventricular function, and there was no evidence of residual ASD shunt or other structural heart diseases. Over the past 2 years, she experienced highly symptomatic AT, and the frequency and duration showed an increasing tendency. Tachycardia was induced and terminated abruptly (Fig. 1A and B). On electrocardiography, P-wave morphology was biphasic in V1; negative in II, III, and aVF; and positive in I and aVL. AT was characterized by P-waves separated by an isoelectric interval, suggesting a focal mechanism of AT (Fig. 1C). Because this tachycardia was only partially responsive to flecainide and β-blockers, we decided to perform catheter ablation. Although focal ectopic AT was suspected as the mechanism, we mapped AT with 3D electroanatomical mapping (NavX, St. Jude Medical, St. Paul, MN, USA) because the patient had a history of cardiac surgery. The tachycardia was found to originate from the anterolateral aspect of the IVC-RA junction, and it showed centrifugal activation with some irregularity during mapping (Fig. 2). There were low-amplitude, fractionated, and double potentials at the earliest activation site (Fig. 3). AT was successfully terminated 5 seconds after a single radiofrequency application (5-mm Blazer II Catheter, EP Technologies Inc., San Jose, CA, USA; 50 W and 60℃), and thereafter, it was not inducible with pacing with or without isoproterenol infusion. The procedure was completed without any complications.
DISCUSSION
AT in patients with surgically corrected congenital heart disease is generally caused by a macro-reentry mechanism.13 However, we described herein a case of focal AT originating from the IVC-RA junction, which was a cannulation site for cardiopulmonary bypass during ASD surgery performed 17 years earlier. The IVC is a rare site of focal atrial arrhythmia.45 Kato, et al.6 reported a case of IVC tachycardia late after cardiopulmonary bypass with IVC cannulation. In the present case, we mapped a similar IVC tachycardia with 3D electroanatomical mapping and ablated it under 3D electroanatomical-mapping guidance. Although there were three case reports regarding focal AT arising from the IVC so far,678 this was the first case report to demonstrate the mechanism of AT originating from IVC using 3D mapping. 3D electroanatomical mapping allows reconstruction of AT mechanisms and represents an advance in the precise localization and ablation of the arrhythmogenic substrates of post-surgical AT.238 Due to overlapping in electrophysiologic characteristics, the question of whether the mechanism of this AT was enhanced automaticity or micro-reentry is unclear. Low-amplitude, fractionated, and double potentials around the ablation target site, as well as reproducible AT induction with critical range of pacing cycle length, favor reentrant mechanisms. However, cycle length variations during tachycardia suggest non-reentrant mechanisms. AT was terminated with a single application of radiofrequency energy.
Although Murphy, et al.9 demonstrated that the development of late AT after surgical ASD repair is low in patients operated during childhood, an arrhythmogenic substrate can be produced with cardiopulmonary bypass cannulation, creating arrhythmogenic muscular sleeves with an electrical connection between the IVC and RA. These findings suggest that patients who undergo cardiopulmonary bypass using vena cava cannulation can present with IVC tachycardia.10
In conclusion, we reported herein a case of IVC tachycardia, presumably related to earlier cardiopulmonary bypass cannulation performed for open-heart surgery. The tachycardia was successfully ablated under 3D electroanatomical-mapping guidance.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download