Abstract
Purpose
Peramivir is the first intravenously administered neuramidase inhibitor for immediate delivery of an effective single-dose treatment in patients with influenza. However, limited data are available on intravenous (IV) peramivir treatment compared to oral oseltamivir for these patients.
Materials and Methods
With a systematic review and meta-analysis, we compared the efficacy of IV peramivir with oral oseltamivir for treatment of patients with seasonal influenza. MEDLINE, EMBASE, and Cochrane Central Register were searched for relevant clinical trials.
Results
A total of seven trials [two randomized controlled trials (RCTs) and five non-randomized observational trials] involving 1676 patients were finally analyzed. The total number of peramivir- and oseltamivir-treated patients was 956 and 720, respectively. Overall, the time to alleviation of fever was lower in the peramivir-treated group compared with the oseltamivir-treated group [mean difference (MD), -7.17 hours; 95% confidence interval (CI) -11.00 to -3.34]. Especially, pooled analysis of observational studies (n=4) and studies of outpatients (n=4) demonstrated the superiority of the peramivir-treated group (MD, -7.83 hours; 95% CI -11.81 to -3.84 and MD, -7.71 hours; 95% CI -11.61 to -3.80, respectively). Mortality, length of hospital stay, change in virus titer 48 hours after admission, and the incidence of adverse events in these patients were not significantly different between the two groups.
Influenza viruses are important global pathogens with an estimated annual attack rate of 5–10% in adults and 20–30% in children, resulting in substantial disease incidence, hospitalization, and mortality.1 The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommended three U.S. Food and Drug Administration (FDA)-approved influenza antiviral agents [oral oseltamivir, inhaled zanamivir, and intravenous (IV) peramivir] for the prevention and control of influenza during the 2015–2016 influenza season.2
Oseltamivir (F. Hoffmann-La Roche, Ltd., Basel, Switzerland) is the antiviral agent most frequently used for the treatment and prevention of influenza, and its use has increased since pandemic influenza A (H1N1) 2009. Because oseltamivir is administered via an oral route, it is often difficult to use in some cases, particularly in young children, patients with aspiration tendency, critically ill patients or patients requiring mechanical ventilation. Therefore, novel or additional effective agents are needed.3
Peramivir (BioCryst Pharmaceuticals Inc., Durham, NC, USA) is an antiviral agent that blocks viral growth by selectively inhibiting neuramidase (NA), an enzyme that releases viral particles from infected cells, in human influenza A and B viruses, and is administered once daily through an IV route.3 It was licensed in Japan and South Korea in 2010.3 In the United States, IV peramivir is still under investigation as an NA inhibitor (NAI), but it was made temporarily available in 2009 for hospitalized patients infected with pandemic influenza A (H1N1) under an Emergency Use Authorization.4 Recent randomized controlled trials (RCTs) demonstrated that IV peramivir showed better clinical efficacy and antiviral activity than a placebo in uncomplicated influenza and was safe and well tolerated.56
Since the 2010s, several RCTs or observational studies (OBSs), comparing the clinical efficacy of peramivir with that of oseltamivir in influenza patients, have been published.78910111213 However, there is a lack of evidence regarding whether IV peramivir or oral oseltamivir should be used for initial treatment in patients with influenza. Accordingly, the purpose of the present study was to compare the clinical efficacy of these two antiviral agents through a systematic review and meta-analysis of data from clinical trials.
To identify potentially relevant articles, a comprehensive search of three electronic databases (MEDLINE, EMBASE, and Cochrane Central Register) up to December 2016 was performed. The search used keywords related to peramivir: BCX-1812; RWJ 270201; oseltamivir; tamiflu; influenza; flu; H1N1; antivirals; and neuramidase inhibitors; search filters provided by SIGN (http://www.sign.ac.uk/methodology/filters.html) were used. There were no language restrictions and the search was limited to human studies. Trials published solely in abstract form were excluded because the methods and results could not be fully analyzed. In addition, we performed a manual search of the references listed in relevant review articles. As this study was a systematic review of published articles, neither informed consent nor ethics approval was required.
A systematic review and meta-analysis were performed on studies that met the following criteria: 1) randomized controlled or observational cohort studies that treated influenza virus infection; 2) comparison of IV peramivir vs. oral oseltamivir; and 3) the presence of clinical outcomes and/or adverse events.
Two pulmonologists (JHL and YHK) independently retrieved potentially relevant studies and reviewed each study according to predefined criteria for eligibility, and finally extracted data. Any disagreement in the process of study selection or data extraction was resolved through consensus. A predefined form was used to extract data from each study. We used only officially published data. Primary outcomes were the time to alleviation of fever after treatment of antiviral agents. We also assessed changes in viral titer, mortality, length of hospital stay and the incidence of adverse events.
As recommended by the Cochrane Collaboration, we used the Newcastle-Ottawa quality assessment scale (NOS) to assess the risk of bias in OBSs.14 NOS uses a star system to evaluate nonrandomized OBSs in the following three domains: selection, comparability and exposure/outcome. Studies that received a star in each domain were considered to be of high quality.
The quality of RCTs was assessed using the Cochrane Handbook for Systematic Reviews of Interventions ‘risk of bias’ tool.15 Risk of bias was assigned to the following domains as ‘low’, ‘high’ or ‘unclear’: sequence generation/allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. Agreement between reviewers was achieved through a consensus.
We analyzed data in Review Manager Software, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Random-effects models were applied. As for dichotomous variables, treatment effects were presented as risk ratios (RRs) with 95% confidence intervals (CIs) via the Mantel-Haenzel method. Statistical estimates for continuous variables were expressed as raw mean differences (MDs). The heterogeneity was assessed using I2 statistics on a scale of 0–100%. I2 >50% indicated a substantial level of between-study heterogeneity. If necessary, we also investigated the influence of an individual study on the overall effect estimates by removing each study in turn to explore the robustness of the pooled effect. Subgroups were analyzed as necessary. A p value <0.05 was considered statistically significant.
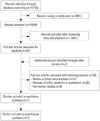
A total of 19155 published articles were initially identified through database searches. After removing duplicate articles, we screened 15554 potentially eligible articles from database searches. Of these articles, 15497 were excluded based on the title and abstract. Therefore, 57 articles remained and two potentially eligible articles were added from their reference lists. A total of 59 articles underwent full-text review. Fifty-two articles were excluded for the reasons presented in Fig. 1. Finally, a total of 7 articles were included in the current analysis.78910111213 Of these trials, two trials were RCTs and the remaining 5 trials were OBSs. All were published between 2011 and 2015. The features of the included studies are shown in Table 1. The number of patients in each trial ranged from 32 to 1091. The total number of patients in our systematic review and meta-analysis was 1676, of whom 956 were treated with IV peramivir and 720 received oral oseltamivir. Quality assessment findings of RCTs and non-randomized OBSs are demonstrated in Fig. 2 and Table 2, respectively.
Fig. 3 shows the effect of IV peramivir and oral oseltamivir on the time to alleviation of influenza symptoms. Overall, a random effect model indicated that the peramivir-treated group had a significantly shorter time to alleviation of influenza symptoms or fever compared with the oseltamivir-treated group (MD, -7.17 hours; 95% CI -11.00 to -3.34; p<0.01; I2 =2%).789101113 Subgroup analyses of RCTs and OBSs were performed. Although a pooled analysis of OBSs demonstrated the superiority of peramivir-treated group (MD, -7.83 hours; 95% CI -11.81 to -3.84; p<0.01; I2 =0%),9101113 that of RCTs did not (MD, 5.86 hours; 95% CI -24.66 to 36.38; p=0.71; I2 =52%).78 Additionally, a subgroup analysis of studies on outpatients showed that the peramivir-treated group had significantly better outcomes than the oseltamivir-treated group (MD, -7.71 hours; 95% CI -11.61 to -3.80; p<0.01; I2 =0%, respectively) in terms of the time to alleviation of fever (Fig. 4B).891011 However, an analysis of studies on hospitalized patients did not reveal a significant difference between groups (MD, 6.22 hours; 95% CI -24.16 to 36.60; p=0.69; I2 =50%) (Fig. 4A).713
Total mortality and length of hospital stay were reported in two trials, respectively.1213 A random effect model showed that total mortality was not significantly different between the peramivir and oseltamivir treatment groups (28.0% vs. 34.2%; RR, 0.96; 95% CI 0.55 to 1.68; p=0.90; I2 =0%) (Fig. 5A).1213 Length of hospital stay in the peramivir-treated group was also similar to that in the oseltamivir-treated group (MD, 0 days; 95% CI -1.19 to 1.20; p=1.00; I2 =0%) (Fig. 5B).712
As shown in Fig. 6A, the incidence of adverse events was not significantly different between peramivir- and oseltamivir-treated groups (72.3% vs. 75.2%; RR, 1.05; 95% CI 0.77 to 1.43; p=0.76; I2 =63%).7813 Pooled estimates also revealed no significant differences in the rates of serious adverse events between the two groups (7.2% vs. 6.8%; RR, 1.06; 95% CI 0.69 to 1.63; p=0.80; I2 =0%) (Fig. 6B).78
Our study showed that IV peramivir might reduce the time to alleviation of fever compared with oral oseltamivir among patients with influenza. The clinical efficacy of IV peramivir therapy was first reported in a placebo-controlled, double-blind phase II study in patients with uncomplicated seasonal influenza.5 At both 300 and 600 mg, a single IV peramivir infusion significantly reduced the time to alleviation of symptoms for adult influenza outpatients.5 After that, a randomized, double-blind phase III study conducted in Japan, Taiwan, and South Korea compared IV peramivir (a 300- or 600-mg single infusion) with oral oseltamivir (75 mg twice a day for 5 days) in uncomplicated cases of seasonal influenza, and demonstrated the non-inferiority of peramivir therapy to oseltamivir in terms of the time to alleviation of symptoms.8 However, due to the scar city of clinical comparisons, we sought to determine which antiviral agent was superior through a meta-analysis of previous trials.
In our study, the time to alleviation of fever was considered a primary outcome when evaluating the clinical efficacy of antiviral agents for patients with influenza because it was regarded as the most important parameter in clinical studies. Although OBSs demonstrated the superiority of IV peramivir therapy with regard to fever, we could not draw concrete conclusions because the results of subgroup analysis from RCTs were ambiguous.
In addition, we compared the time to alleviation of fever according to whether patients were hospitalized or not. Although the US FDA approved peramivir as the first IV NAI for patients with uncomplicated influenza,16 a recent placebo-controlled, double-blind RCT did not demonstrate the clinical benefit of IV peramivir in hospitalized patients with influenza.17 No antiviral agents have been approved specifically for the treatment of influenza in hospitalized patients. In our meta-analysis, there were no statistically significant differences between treatment groups in hospitalized patients with influenza. Therefore, we could not confirm whether IV peramivir or oral olsetamivir was more effective in patients with serious influenza requiring hospitalization.
Total mortality, length of hospital stay, and changes in viral titers are key parameters of the clinical and virological effectiveness of antiviral agents. Our results revealed that these parameters did not differ between peramivir and olsetamivir treatment groups. In a RCT that enrolled 288 healthy volunteers (aged 18–45 years) intranasally inoculated with experimental influenza A or B, oral peramivir treatment at a dosage of 400 mg once daily for 5 days significantly reduced viral detection, defined by the area under the curve for nasal wash viral titers of influenza A.18 And both 400 and 800 mg once daily for 5 days reduced viral titer of influenza B.18 Although we evaluated changes in viral titers from base-line to 48 hours, no significant difference in virological effects was found between two groups.
In addition to clinical efficacy, adverse events are critical factors in the selection of an antiviral agent. Although we conducted a meta-analysis of adverse events using two RCT studies and one OBS,7813 the results showed no statistically significant differences in rates of any or serious adverse events between patients treated with peramivir and those treated with oseltamivir. Most adverse events were mild or moderate. Pooled estimated results suggested that the incidence of severe adverse events was similar in the peramivir and oseltamivir groups (7.2% and 6.8%, p=0.08). Gastrointestinal symptoms such as diarrhea, nausea, and vomiting were the most common adverse effects in both treatment groups. The similar clinical efficacy and adverse event findings of this systematic review suggest that the choice between oseltamivir and peramivir therapy could be decided based upon the convenience of administration (IV vs. oral), the ease with which medications can be purchased, and the preference of the patient or physician. Several previous OBSs and subgroup analysis in our study showed IV peramivir to have superior efficacy in terms of the time to alleviation of fever. However, the lack of differences in total mortality rate, length of hospital stay and changes in viral titers indicate little or no difference in clinical efficacy between the two agents.
The development of influenza antiviral drug resistance in viruses is a major concern. Therefore, when considering which drug to choose, resistance should be considered. The H274Y mutation is associated with resistance to oseltamivir and peramivir and mutations at I222 and R292 can reduce peramivir sensitivity.19 The incidence of resistance to oseltamivir and/or peramivir in Japan and USA during the 2013–2014 influenza season was 4.2% and 1.2% for influenza A (H1N1) pdm09, respectively. This might mean that, in spite of the low resistance rate, a particular drug could be superior to others in specific situations.
Our study has some limitations. First, since our meta-analysis considered only a small number of trials, our results should be interpreted with caution. Second, the publication bias inherent to all meta-analyses might have influenced these results. However, since the number of included trials was small, we could not estimate potential publication bias with a funnel plot for all outcomes. Third, the dosage and the duration of IV peramivir treatment varied among studies, which could affect the precision of the results. As a result, we think that additional large-scale RCTs are needed to overcome these limitations. Finally, we tried to compare the efficacy of two drugs between children and adults. We found six studies for primary outcome.789101113 There are three studies for adults,7813 and one study for children.9 The remaining two studies examined mixed group including adults and children.1011 Accordingly, we could not perform a meta-analysis for children group. Instead, we evaluated the time to alleviation of fever after treatment of antiviral agents on adults group.7813 Pooled estimates revealed no significant difference between two treatments (MD, -2.51 hours; 95% CI -11.88 to 6.86; p=0.60; I2 =6%).
In conclusion, our systematic review and meta-analysis suggested that IV peramivir might reduce the time to alleviation of fever among patients with influenza compared to oral oseltamivir. However, because the methodological limitations of the included trials and the scarcity of trials prevented us from drawing firm conclusions, the clinical benefit and/or superiority of IV peramivir to oral oseltamivir remains unclear in these patients. Accordingly, further large-scale RCTs are needed to establish appropriate criteria with regard to the selection of optimal NAIs for patients with influenza.
Figures and Tables
 | Fig. 2Risk of bias summary (A) and risk of bias graph (B) for randomized controlled studies included in this meta-analysis. |
 | Fig. 3Pooled adjusted risk ratio results for time to alleviation of fever among patients with influenza treated with intravenous peramivir versus oral oseltamivir in randomized controlled trials (A) and observational studies (B). SD, standard difference; IV, inverse variance; CI, confidence interval; df, degrees of freedom. |
 | Fig. 4Pooled adjusted risk ratio results for time to alleviation of fever with intravenous peramivir versus oral oseltamivir in inpatients (A) and outpatients (B) with influenza. SD, standard difference; IV, inverse variance; CI, confidence interval; df, degrees of freedom. |
 | Fig. 5Pooled adjusted odds ratio results for secondary among patients with influenza treated with intravenous peramivir versus oral oseltamivir. Mortality (A), length of hospital stay in days (B), and changes in viral titers from baseline (C) to 48 hours. M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom; SD, standard difference; IV, inverse variance. |
 | Fig. 6Pooled analysis of adverse events among patients with influenza treated with intravenous peramivir versus oral oseltamivir. All adverse events (A) and serious adverse events (B). M-H, Mantel-Haenszel; CI, confidence interval; df, degrees of freedom. |
Table 1
Characteristics of the Studies Included in the Meta-Analysis

| Study | Design | Study period | Total patients (no.) | Age (mean) | Age group | Male (%) | Treatment location | Identified influenza virus subtype | Intervention protocol | Major outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||||
| Ison, et al.7 | Multinational, multicenter, double-blind | July 2007–September 2008 | 137 | 59.3 | Adult | 46.7 | Hospital | A (H1N1), A (H3N2), B | 5-day treatment with intravenous peramivir once daily vs. oral oseltamivir twice daily | Time to clinical stability, time to alleviation of symptoms, time to discharge, the change in influenza virus titer, adverse events |
| Kohno, et al.8 | Multinational, multicenter, double-blind, double-dummy | November 2008–April 2009 | 1091 | 35.1 | Adult | 51.5 | - | A (H1), A (H3), B | Single-dose intravenous peramivir vs. oral oseltamivir twice daily for 5 days | Time to alleviation of influenza symptoms, the change in influenza virus titer, adverse events |
| Observational studies | ||||||||||
| Hikita, et al.9 | Two-center, prospective single-arm study | February 2011–April 2011 | 223 | 6.4 | Child | 55.1 | Outpatient clinics | A, B | Single-dose intravenous peramivir vs. other neuramidase inhibitors (oral oseltamivir twice daily for 5 days or single-dose inhaled laninamivir, or inhaled zanamivir twice daily for 5 days) | Time to alleviation of fever |
| Shobugawa, et al.10 | Two-center, retrospective single-arm study | December 2010–March 2011 | 108 | 5.2 | Mix | 51.8 | Outpatient clinics | A (H3N2) | Oral oseltamivir twice daily for 5 days or inhaled zanamivir twice daily for 5 days or a single inhaled laninamivir once, or a single intravenous peramivir once | Time to alleviation of influenza symptoms |
| Takemoto, et al.11 | Multicenter, retrospective single-arm study | November 2012–March 2013 | 104 | 27.0 | Mix | 55.7 | Outpatient clinics | A, B | Oral oseltamivir twice daily for 5 days or inhaled zanamivir twice daily for 5 days or single-dose inhaled laninamivir, or single-dose intravenous peramivir | Time to alleviation of fever |
| Yoo, et al.12 | Single-center, retrospective cross-over study | December 2010–March 2014 | 60 | 68.0 | Adult | 55.0 | Intensive care unit | A, B | Intravenous peramivir once daily for a median of 6 days vs. oral oseltamivir twice daily for a median of 5.5 days | Clinical complications, management, and clinical outcomes |
| Yoshino, et al.13 | Single-center, retrospective single-arm study | October 2012–March 2013 | 32 | 75.5 | Adult | 56.0 | Hospital | - | Single-dose intravenous peramivir vs. oral oseltamivir twice daily for 5 days | Time to defervescence and survival rate |
Table 2
Risk of Bias within Non-Randomized Trials Using Newcastle-Ottawa Scale

| Study | Selection | Comparability | Outcome of interest | Overall quality | |||||
|---|---|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cohorts | Outcome assessment | Same methods of ascertainment for cases and controls | Non-response rate | ||
| Hikita, et al.9 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | NA | High |
| Shobugawa, et al.10 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | NA | High |
| Takemoto, et al.11 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | NA | High |
| Yoo, et al.12 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | NA | High |
| Yoshino, et al.13 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | NA | High |
ACKNOWLEDGEMENTS
This research was supported by the 2017 scientific promotion program funded by Jeju National University.
References
1. Vaccines against influenza WHO position paper–November 2012. Wkly Epidemiol Rec. 2012; 87:461–476.
2. Centers for Diseases Control and Prevention. Vaccine Recommendations of the ACIP. accessed on 2016 December 27. Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/flu.html.
3. Wester A, Shetty AK. Peramivir injection in the treatment of acute influenza: a review of the literature. Infect Drug Resist. 2016; 9:201–214.
4. Birnkrant D, Cox E. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009; 361:2204–2207.

5. Kohno S, Kida H, Mizuguchi M, Shimada J. S-021812 Clinical Study Group. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother. 2010; 54:4568–4574.

6. Kohno S, Kida H, Mizuguchi M, Hirotsu N, Ishida T, Kadota J, et al. Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob Agents Chemother. 2011; 55:2803–2812.

7. Ison MG, Hui DS, Clezy K, O'Neil BJ, Flynt A, Collis PJ, et al. A clinical trial of intravenous peramivir compared with oral oseltamivir for the treatment of seasonal influenza in hospitalized adults. Antivir Ther. 2013; 18:651–661.

8. Kohno S, Yen MY, Cheong HJ, Hirotsu N, Ishida T, Kadota J, et al. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother. 2011; 55:5267–5276.

9. Hikita T, Hikita H, Hikita F, Hikita N, Hikita S. Clinical effectiveness of peramivir in comparison with other neuraminidase inhibitors in pediatric influenza patients. Int J Pediatr. 2012; 2012:834181.

10. Shobugawa Y, Saito R, Sato I, Kawashima T, Dapat C, Dapat IC, et al. Clinical effectiveness of neuraminidase inhibitors--oseltamivir, zanamivir, laninamivir, and peramivir--for treatment of influenza A(H3N2) and A(H1N1)pdm09 infection: an observational study in the 2010-2011 influenza season in Japan. J Infect Chemother. 2012; 18:858–864.

11. Takemoto Y, Asai T, Ikezoe I, Yano T, Ichikawa M, Miyagawa S, et al. Clinical effects of oseltamivir, zanamivir, laninamivir and peramivir on seasonal influenza infection in outpatients in Japan during the winter of 2012-2013. Chemotherapy. 2013; 59:373–378.

12. Yoo JW, Choi SH, Huh JW, Lim CM, Koh Y, Hong SB. Peramivir is as effective as oral oseltamivir in the treatment of severe seasonal influenza. J Med Virol. 2015; 87:1649–1655.

13. Yoshino Y, Seo K, Koga I, Kitazawa T, Ota Y. Clinical efficacy of peramivir in adult patients with seasonal influenza during the winter of 2012 in Japan. Clin Respir J. 2015; 9:228–232.

14. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if non-randomized studies in meta-analyses. accessed on 2016 December 27. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.

16. Alame MM, Massaad E, Zaraket H. Peramivir: a novel intravenous neuraminidase inhibitor for treatment of acute influenza infections. Front Microbiol. 2016; 7:450.

17. de Jong MD, Ison MG, Monto AS, Metev H, Clark C, O'Neil B, et al. Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clin Infect Dis. 2014; 59:e172–e185.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download