Abstract
Congenital erythrocytosis (CE) is a rare and heterogeneous disease. The high oxygen affinity hemoglobin (Hb) variants are the most common cause of CE. Herein, we report a Korean patient with isolated erythrocytosis. A 25-year-old man was referred to our hospital for evaluation of high Hb level (Hb 20.4 g/dL, hematocrit 58%, reticulocyte count 2.90%, white blood cell count 6.83×109/L, and platelet count 195×109/L). Bone marrow biopsy revealed normocellular marrow without myeloproliferative features. JAK2 (V617F, exon 12), CALR (exon 9), and MPL W515K/L mutations were not detected. P50 (partial pressure at which Hb is half saturated with oxygen), which is an indicator of left-shift of oxygen dissociation curve (high oxygen affinity state), was 14.3 mm Hg (reference value 22.6–29.4 mm Hg). He was suspected to have CE. Mutation analysis of the HBB gene revealed the known Hb variant, Hb Heathrow [β103(G5)Phe→Leu]. This is the first report of Hb Heathrow in Asian.
Congenital erythrocytosis (CE) can be classified as primary or secondary. Primary CE or primary familial and congenital polycythemia (PFCP) is caused by mutations of the erythropoietin receptor gene (EPOR),1 whereas secondary CE is caused by high oxygen affinity hemoglobin (Hb) variants, 2,3-bisphosphoglycerate (2,3-BPG) deficiency, and dysregulation of hypoxia-sensing pathway.1 More than 90 high oxygen affinity Hb variants have been reported in CE (Database of Human Hemoglobin Variants and Thalassemia mutations; http://globin.bx.psu.edu/hbvar/menu.html). The prognosis and treatment of CE and myeloproliferative neoplasm, Polycythemia vera (P. vera), are different.234 Therefore, differentiation of CE from P. vera is important.
Herein, we describe the Hb variant, Hb Heathrow [β103(G5)Phe→Leu], found in a Korean patient with isolated erythrocytosis.
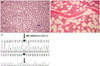
A 25-year-old man was referred from a local hospital for evaluation of high levels of Hb and management of gout. There was no hepatosplenomegaly on physical examination. A complete blood count showed the following results: Hb 20.4 g/dL, hematocrit 58%, reticulocyte count 2.90%, white blood cell count 6.83×109/L, and platelet count 195×109/L. Bone marrow biopsy indicated no definite evidence of myeloproliferative features (Fig. 1A and B). Mutations were not found in JAK2 V617 or exon 12, MPL W515K/L, CALR exon 9. Serum EPO level was within the normal range, 7.3 mIU/mL (reference range: 4.3–29.0 mIU/mL). No abnormalities were found on Hb electrophoresis. P50 (partial pressure at which Hb is half saturated with oxygen), which is an indicator of left-shift of oxygen dissociation curve (high oxygen affinity state) was calculated to be 14.3 mm Hg (reference value 22.6–29.4 mm Hg), using venous blood gas analysis (recommended by international federation of clinical chemistry).15 Congenital (familial) erythrocytosis was suspected. We performed alpha 1-globin gene (HBA1)/alpha 2-globin gene (HBA2), beta-globin gene (HBB), and Von Hippel Lindau (VHL) gene mutation analysis according to a previous study.6 All coding sequences and flanking intronic regions of HBA1/HBA2, HBB, and VHL were analyzed. Sequencing was performed by using the BigDye Terminator Cycle Sequencing Ready Reaction Kit on the ABI Prism 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequence variation was described according to guidelines of the Human Genome Variation Society (http://www.hgvs.org/mutnomen). In case of HBB, the conventional numbering system was also used (Database of Human Hemoglobin Variants and Thalassemia mutations).
More than 90 high oxygen affinity Hb variants have been reported until now (Database of Human Hemoglobin Variants and Thalassemia mutations). High oxygen affinity Hb variants are inherited in an autosomal dominant pattern. The HBB mutation is more prevalent than HBA1/HBA2 mutation.8 High oxygen affinity Hb variants are derived mostly via three mechanisms; mutation affecting transition of R-state (relaxed binding structure, high oxygen affinity) to T-state (tight binding structure, low oxygen affinity), mutation in the 2,3-BPG binding site, and mutation in the heme pocket.1 Hb Heathrow [β103 (G5)Phe→Leu] and Hb Saint Nazaire [β103(G5)Phe→Ile] are examples of mutation in the heme pocket.79 White, et al.7 reported a first case of Hb Heathrow in English family. Patients with Hb Heathrow showed high Hb level (15.7–21.0 g/dL), normal platelet count, normal white blood cell count and low P50.710
The diagnostic strategies for CE have been suggested in several studies. The acquired secondary (pulmonary, renal, cardiac, etc.) and acquired primary erythrocytosis (P. vera; JAK2 mutation with low serum EPO level) are excluded.1 Low serum EPO level with negative JAK2 mutation is suggestive of PFCP.8 In cases of low P50 (<22.6 mm Hg) (high oxygen affinity state), HBA1/HBA2, HBB, and BPGM gene sequencing analyses are recommended.1 VHL, EPAS1, and EGLN1 gene sequencing analyses must be performed in patients with normal serum EPO level.14 However, the clinician must keep in mind that the incidence of erythrocytosis without a known genetic cause (idiopathic erythrocytosis) is surprisingly high.8 For example, Bento, et al.8 reported that only 25 of 70 patients (36%) had a known causative gene defect. Similarly, only 13.2 % of Korean patients with erythrocytosis had a known causative gene mutation.6 Therefore, further studies are needed to identify the new genetic cause of erythrocytosis.
In conclusion, we reported first case of Hb Heathrow in Asian. High oxygen affinity Hb variant is a common cause of CE. Therefore, the presence of a high oxygen affinity Hb variant must be ruled out, especially in a case of erythrocytosis with normal/high serum EPO level and low P50.
Figures and Tables
Fig. 1
Peripheral blood, bone marrow section, and HBB sequencing analysis results. (A) Erythrocytosis (peripheral blood, Wright-Giemsa, ×400) and (B) normocellular marrow without myeloproliferative features are noted (hematoxylin and eosin stain, ×100). (C) DNA sequencing of hemoglobin beta gene identified a heterozygous mutation at position 310, resulting in replacement of a phenylalanine by a leucine residue [β103(G5)Phe→Leu, NM_000518.4:c.310T>C (p.Phe104Leu)]. The arrow indicates the 310th nucleotide. HBB, beta-globin gene.
References
1. Bento C, Percy MJ, Gardie B, Maia TM, van Wijk R, Perrotta S, et al. Genetic basis of congenital erythrocytosis: mutation update and online databases. Hum Mutat. 2014; 35:15–26.

2. Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014; 124:2507–2513.

3. Thom CS, Dickson CF, Gell DA, Weiss MJ. Hemoglobin variants: biochemical properties and clinical correlates. Cold Spring Harb Perspect Med. 2013; 3:a011858.

4. Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004; 103:3924–3932.

5. Burnett RW, Covington AK, Fogh-Andersen N, Külpmann WR, Maas AH, Müller-Plathe O, et al. International Federation of Clinical Chemistry (IFCC), Committee on pH, Blood Gases and Electrolytes: approved IFCC recommendation on definitions of quantities and conventions related to blood gases and pH. Eur J Clin Chem Clin Biochem. 1995; 33:399–404.
6. Jang JH, Seo JY, Jang J, Jung CW, Lee KO, Kim SH, et al. Hereditary gene mutations in Korean patients with isolated erythrocytosis. Ann Hematol. 2014; 93:931–935.

7. White JM, Szur L, Roberts P, Lorkin PA, Lehmann H. Hb heathrow: beta G5 103 phenylalanine-leucine: a new high affinity haemoglobin. Br J Haematol. 1973; 25:284.
8. Bento C, Almeida H, Maia TM, Relvas L, Oliveira AC, Rossi C, et al. Molecular study of congenital erythrocytosis in 70 unrelated patients revealed a potential causal mutation in less than half of the cases (Where is/are the missing gene(s)?). Eur J Haematol. 2013; 91:361–368.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download