Abstract
Purpose
This study examined 2-year outcome of consecutive therapy using entecavir (ETV) followed by telbivudine (LdT) in subjects with undetectable hepatitis B virus (HBV) DNA level and normal alanine aminotransferase level after the initial 6 months of ETV treatment.
Materials and Methods
Sixty subjects were randomized to continue with ETV or switch to LdT. Significant difference in baseline characteristics was not found between the two groups. Persistent HBV DNA level of 20–60 IU/mL in three consecutive samples collected three months apart or singly measured HBV DNA level of >60 IU/mL was defined as virological rebound.
Results
During 96 weeks of follow-up, all subjects of the ETV-only group (n=30) resulted in undetectable HBV DNA level. On the other hand, 83.3% (n=25) of the LdT-switched group showed treatment success. Virological rebound time varied from week 24 to 84 after switching to LdT. HBV DNA level was 180 to 2940 IU/mL at rebound time. All subjects with virological rebound (n=5) showed drug-resistant mutation: three had mutation rtM204I, and two had mutation rtM204V. Consecutive treatment using ETV followed by LdT showed virological rebound in 16.7% of subjects during 96 weeks of follow-up. HBV DNA negativity during initial ETV therapy could not be achieved in patients who switched to LdT.
Conclusion
Consecutive treatment using ETV followed by lamivudine was ineffective for treating chronic hepatitis B. LdT was found as a more potent antiviral agent than lamivudine. However, this conclusion requires larger-scale, long-term prospective reviews of the treatment effects of ETV-LdT switch therapy.
About 400 million people in the world are infected with hepatitis B virus (HBV), which can greatly affect the health of these population. Chronic hepatitis B (CHB) is linked to liver cirrhosis and hepatocellular carcinoma.1 Several nucleoside and nucleotide analogs are used in CHB treatment. Currently, entecavir (ETV) and tenofovir are generally considered first-line treatments for treatment-naïve patients, since these drugs have relatively strong antiviral effects and low incidences of resistance.2 However, identification of other treatment options for patients with CHB infection is important, because many CHB hepatitis patients cannot afford antiviral therapy due to the high cost of these drugs.
A cost-effective34 possible alternative treatment involves initial treatment with a potent antiviral agent with a low incidence of resistance, then changing to a more economical agent for maintenance therapy once HBV DNA negativity is achieved. Fung, et al.5 investigated the virological outcomes of patients with HBV DNA negativity induced by ETV who switched to lamivudine: the study examined if HBV DNA loss was maintained during a 2-year treatment period, and they showed that switching therapy from ETV to lamivudine resulted in virological rebound in 24% of patients. Thus, lamivudine switch showed a high possibility of antiviral resistance even when HBV DNA loss was achieved. Rebound due to mutations is also a possibility when the drug is taken for a long time.67 We observed mutations in approximately 30% of HBV DNA-negative patients (unpublished data), who had maintained this negative status for more than 3 years. Therefore, despite its cost-effectiveness, long-term treatment of lamivudine appears to be insufficient for maintaining HBV DNA negativity.
Telbivudine (LdT) is less potent than ETV or tenofovir but is more economical. Some studies showed that LdT is superior to long-term lamivudine after achieving the loss of HBV DNA because of its stronger antiviral potency and lower incidence of resistance.89 We, therefore, evaluated the effectiveness of ETV-LdT switch therapy in CHB patients who achieved the loss of HBV DNA and normal liver function through initial ETV therapy.
We prospectively recruited 60 patients from National Health Insurance Service Ilsan Hospital, in Goyang, Korea, between July 2011 to December 2012. Patients eligible for entry to this study were age ≥18 years and treated with ETV 0.5 mg for at least 6 months, with a normal alanine aminotransferase (ALT; ≤40 IU/L) and undetectable HBV DNA [<20 IU/mL with a polymerase chain reaction (PCR) assay]. Then, the patients were assigned randomly to two groups by the primary investigator in a 1:1 ratio to two arms using computer-generated numbers preassigned to either arm. The first group (ETV-ETV) continued on ETV 0.5 mg daily, whereas patients in the second group (ETV-LdT) were switched from ETV to LdT 600 mg daily. Exclusion criteria included evidence of hepatocelluar carcinoma (HCC), a history of decompensated liver cirrhosis, co-infection with hepatitis C, hepatitis D, or HIV. The study protocol conformed to the guidelines of the 1975 Declaration of Helsinki and was approved by our Institutional Review Board.
Serum HBV DNA level, hepatitis B serology, ALT level, liver function test results, and hematology results such as complete blood count with differential analysis were evaluated every 3 months after randomization. Serum HBV DNA level was measured using the COBAS Amplicor Monitor 2.0 HBV assay (Roche Molecular Systems, Branchburg, NJ, USA; low detection limit <20 IU/mL). Analysis for drug-resistant mutations was performed at the time of virological breakthrough. Breakthrough was determined by line-probe assays using INNO-LiPA HBV DR v2 and v3 (Innogenetics, Ghent, Belgium), allowing simultaneous detection of wild-type HBV polymerase and drug-induced mutations associated with lamivudine (LAM) and ETV resistance at codons rt169, 173, 180, 184, 202, 204, and 250 of the HBV polymerase region. HBeAg was measured using standard enzyme-linked immunosorbent assays (Abbott Diagnostics, Abbott Park, IL, USA). The upper normal limit of ALT was 40 IU/L.
Virological rebound was defined as a single HBV DNA level of >60 IU/mL or persistent HBV DNA level of 20–60 IU/mL for 3 consecutive samples collected 3 months apart. Additional mutational analyses were performed on patient samples at the time of virological rebound.
Cirrhosis was defined as either platelet count <100000/L with ultrasonographic findings suggestive of cirrhosis including a blunted, nodular liver edge accompanied by splenomegaly (>12 cm), esophageal or gastric varices; or overt complications of liver cirrhosis including ascites, variceal bleeding, and hepatic encephalopathy.
Patients who developed virological rebound were switched back to and continued on ETV for the remainder of the follow-up period. Patients with LdT resistance were treated with tenofovir.
Statistical analyses were performed with SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as median (range) or number (%). Chi-square test was used for categorical variables and Fisher's exact test when necessary. For continuous variables, differences were evaluated with a t-test. p values less than 0.05 were considered statistically significant.
A total of 60 patients were enrolled and were evenly allocated into two groups: ETV maintenance (ETV-ETV, n=30) or LdT switch (ETV-LdT, n=30). The baseline characteristics of the two groups are shown in Table 1. The median age was 54.0 years for ETV-ETV and 52.0 years for ETV-LdT, and the median duration of prior ETV therapy was 21.0 months (ETV-ETV group) and 24.0 months (ETV-LdT group). Cirrhosis was present in 11 patients (36.7%) in the ETV-ETV group and 10 (33.3%) in the ETV-LdT group. No statistical differences were observed between the two groups for age, gender, HBeAg status, or liver biochemistry.
All patients had normal ALT level at the beginning of the study. During scheduled visits at weeks 12, 24, 48, 72, 84, and 96, no patients showed ALT level above the upper normal range.
In the ETV-ETV group, no evidence of virological rebound was observed during the 96-week follow-up. In ETV-LdT group, five patients developed virological rebound (Fig. 1); characteristics of these patients are shown in Table 2. Virological rebound time was variable at weeks 24 to 84 after switching to LdT. The HBV DNA level at virological rebound varied from 180 to 2940 IU/mL. All five patients with virological rebound had evidence of drug-resistant mutations, with three patients with the rtM204I mutation and two with the rtM204V mutation. Patient 1 showed HBV DNA level of 35 IU/mL at week 60 and 34 IU/mL at week 72 after switching to LdT; however, this level reached 180 IU/mL at week 84 (Fig. 2). Patient 2 maintained HBV DNA loss up to 60 weeks after switching to LdT, but serum HBV DNA level increased to 600 IU/mL at week 72. Patient 3 showed HBV DNA of 30 IU/mL and 39 IU/mL at weeks 24 and 36 after switching to LdT, respectively. However, at week 48, serum HBV DNA level increased to 2940 IU/mL. In patients 4 and 5, serum HBV DNA level increased to 2680 IU/mL at week 48 and 1350 IU/mL at week 24, respectively, without prior HBV DNA increase. These 5 patients were subsequently changed to tenoforvir and had undetectable HBV DNA 12 weeks later.
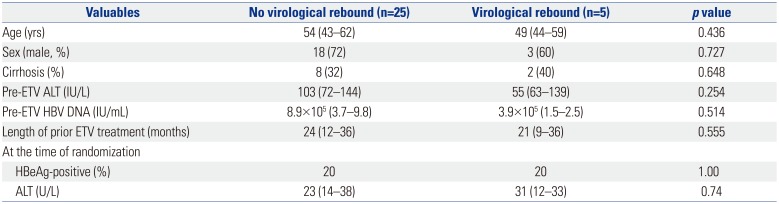
The other 25 patients in the ETV-LdT group maintained undetectable HBV DNA level and normal liver function during 96 weeks of treatment. Age, gender, HBeAg, serum ALT level, serum HBV DNA level before switching, and initial ETV treatment period were not significantly related to virological rebound (Table 3).
Most studies of antiviral agent change have focused on antiviral resistance and insufficient effects of the chosen antiviral agent. Furthermore, these studies were conducted on monoexclusive or combination therapy of antiviral agents applicable for controlling resistance.101112131415 However, little research has addressed switching antiviral agents to nucleoside or nucleotide analogs in circumstances without resistance, except for studies on the sequential therapy of oral antiviral agents and interferon.1617 In particular, few reports have studied the effects of switching to a more cost-effective agent after complete virogical response was achieved with a potent but more expensive antiviral agent.5
Lack of evidence on this topic may be due to the notion that long-term treatment with a low-cost, low-potency agent with a high incidence of resistance may result in treatment failure. However, resistance to lamivudine develops in approximately 15–20% of patients per year, with 5.0% resistance rates to LdT in the first year and 25.1% in the second year of treatment in HBeAg-positive patients. Although the incidence of resistance to LdT therapy in CHB patients is lower than resistance to lamivudine, these levels are still too high to be overlooked when compared to ETV or tenofovir.18
Fung, et al.5 started treating patients with HBV DNA loss with ETV and then switched to lamivudine and continued the treatment for 2 years. The study was based on an earlier study that reported a low incidence of resistance in the first 2 years of lamivudine treatment in patients with serum HBV DNA that was undetectable with PCR (<300 copies/mL) at week 24 of lamivudine treatment.7 Viral reemergence was observed in 6 of 25 patients (24%), and resistance was found in 3. Therefore, Fung, et al.5 expressed negative views toward lamivudine-switching therapy in patients with a favorable response to ETV therapy. However, they also reported that, after rebound, 3 patients were again treated with ETV to induce HBV DNA loss. Of the 3 patients who developed lamivudine resistance, 1 did not properly take the drug, while another had a history of lamivudine that could have affected resistance development. If patients with appropriate medical history are chosen and serum HBV DNA level is monitored after return to ETV immediately after HBV DNA rebound, a switch to lamivudine therapy might be acceptable.
Reports on LdT indicate that serum HBV DNA and ALT levels before treatment and degree of viral suppression at week 24 can be used as markers to predict treatment response through week 96.3 In HBeAg-positive patients whose initial serum HBV DNA was less than 109 copies/mL, initial serum ALT was twice the upper normal limit, and serum HBV DNA at week 24 was undetectable (less than 300 copies/mL), the rate of serum HBV DNA loss was 89% after 2 years, with a resistance incidence of 1.8%. In HBeAg-negative patients, when initial serum HBV DNA was less than 107 copies/mL, initial ALT was twice the upper normal limit, and serum HBV DNA at week 24 was undetectable, serum HBV DNA level was consistently not detected in 91% after 2 years, with a resistance incidence of 2.3%.3 This incidence is noticeably lower than the overall resistance incidence for LdT.
Therefore, our study evaluated the effect of ETV-LdT switch therapy. We used LdT because its cost is similar to that of lamivudine, but it is considered more effective than lamivudine.8919 However, our results showed virological breakthrough in 5 of 30 patients (16.6%) who were switched from ETV to LdT, and resistant mutations were found in all of these patients.
In contrast to ETV-lamivudine switch therapy, where resistance mutations were observed in only 50% of patients with virological rebound, genotypic resistance in this study was found in all patients. Therefore, a switch back to ETV 0.5 mg was not considered. The incidence of resistance in this study was higher than the rate for LdT treatment of naïve patients3 and was similar to the incidence of resistance seen with ETV-lamivudine switch therapy.5 In the present study, none of the patients who developed resistance after switching therapy had a medical history of treatment with antiviral agents or poor compliance, as had been found in a previous study of ETV-lamivudine switch therapy, and we identified no significant risk factors. The only difference between patients with and without virological breakthrough was a lower ALT level before initial ETV treatment in those with breakthrough compared to those who maintained HBV DNA negativity after switching to LdT. This result suggests that the patient's degree of immune activity before beginning ETV might affect virological response after switching from ETV to LdT. Also, it is possible that the ETV that was used before affected the development of resistance, although we didn't evaluate the presence of resistance mutations before the study begun. Mutations had already developed in 204 areas of HBV DNA polymerase during ETV treatment, but the high antiviral potency and resistance barrier of ETV prevented virological rebound and maintained HBV DNA negativity. Switching to LdT, which has a lower potency and tolerance barrier, may have allowed the mutation to have an effect.
Compared to ETV-lamivudine switch therapy, ETV-LdT switch therapy did not show the superiority of maintaining HBV DNA negativity. Therefore, it is not recommended to switch from ETV to LdT for cost-effectiveness in patients with HBV DNA negativity resulted from initial ETV therapy.
The use of tenofovir, which is effective in treating patients who are resistant to nucleoside analogs including LdT, is clinically approved. Even if resistant mutations develop after sw-itching to LdT, appropriate control of resistance will be possible if virological breakthrough is identified early and treated with tenofovir. Although our results were not significant due to small number of patients, investigating factors that affect the maintenance of complete virological response in ETV-LdT switch therapy is needed to support patient treatment selections. Long-term treatment should be considered in patients with chronic HBV, which is an economic burden. This conclusion should be consolidated with larger-scale, long-term prospective reviews of the treatment effects of ETV-LdT switch therapy.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Health Insurance Service Ilsan Hospital, Republic of Korea.
References
2. Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in ‘real-life’ settings: from clinical trials to clinical practice. J Viral Hepat. 2012; 19:377–386. PMID: 22571899.

3. Zeuzem S, Gane E, Liaw YF, Lim SG, DiBisceglie A, Buti M, et al. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009; 51:11–20. PMID: 19345439.
4. Yuen MF, Fong DY, Wong DK, Yuen JC, Fung J, Lai CL. Hepatitis B virus DNA levels at week 4 of lamivudine treatment predict the 5-year ideal response. Hepatology. 2007; 46:1695–1703. PMID: 18027877.

5. Fung J, Lai CL, Yuen J, Cheng C, Wu R, Wong DK, et al. Randomized trial of lamivudine versus entecavir in entecavir-treated patients with undetectable hepatitis B virus DNA: outcome at 2 Years. Hepatology. 2011; 53:1148–1153. PMID: 21480321.

6. Lee HW, Lee HJ, Hwang JS, Sohn JH, Jang JY, Han KJ, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology. 2010; 51:415–421. PMID: 19902424.

7. Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001; 34(4 Pt 1):785–791. PMID: 11584376.

8. Lai CL, Leung N, Teo EK, Tong M, Wong F, Hann HW, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005; 129:528–536. PMID: 16083710.

9. Lui YY, Tsoi KK, Wong VW, Kao JH, Hou JL, Teo EK, et al. Cost-effectiveness analysis of roadmap models in chronic hepatitis B using tenofovir as the rescue therapy. Antivir Ther. 2010; 15:145–155. PMID: 20386069.

10. Lee JM, Kim HJ, Park JY, Lee CK, Kim DY, Kim JK, et al. Rescue monotherapy in lamivudine-resistant hepatitis B e antigen-positive chronic hepatitis B: adefovir versus entecavir. Antivir Ther. 2009; 14:705–712. PMID: 19704174.
11. Leemans WF, Janssen HL, Niesters HG, de Man RA. Switching patients with lamivudine resistant chronic hepatitis B virus from tenofovir to adefovir results in less potent HBV-DNA suppression. J Viral Hepat. 2008; 15:108–114. PMID: 18184193.

12. Seo YS, Kim JH, Yeon JE, Park JJ, Kim JS, Byun KS, et al. Antiviral efficacy of adefovir dipivoxil versus lamivudine in patients with chronic hepatitis B sequentially treated with lamivudine and adefovir due to lamivudine resistance. World J Gastroenterol. 2007; 13:4072–4079. PMID: 17696224.
13. Fung J, Lai CL, Yuen JC, Wong DK, Tanaka Y, Mizokami M, et al. Adefovir dipivoxil monotherapy and combination therapy with lamivudine for the treatment of chronic hepatitis B in an Asian population. Antivir Ther. 2007; 12:41–46. PMID: 17503746.
14. Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007; 133:1445–1451. PMID: 17983801.

15. Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006; 130:2039–2049. PMID: 16762627.

16. Niro GA, Fontana R, Gioffreda D, Fiorella S, Accadia L, Iacobellis A, et al. Sequential treatment with lamivudine and alpha-interferon in anti-HBe-positive chronic hepatitis B patients: a pilot study. Dig Liver Dis. 2007; 39:857–863. PMID: 17652045.

17. Shi M, Wang RS, Zhang H, Zhu YF, Han B, Zhang Y, et al. Sequential treatment with lamivudine and interferon-alpha monotherapies in hepatitis B e antigen-negative Chinese patients and its suppression of lamivudine-resistant mutations. J Antimicrob Chemother. 2006; 58:1031–1035. PMID: 16987866.
18. Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, et al. 2-year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009; 136:486–495. PMID: 19027013.

19. Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007; 357:2576–2588. PMID: 18094378.

Fig. 1
Study design and patient flow for both arms. A total of 60 patients with CHB were enrolled. Study population has been treated with 0.5 mg ETV for at least six months. A total of 60 patients were assigned randomly to ETV monotherapy continued group and LdT monotherapy switch group in 1:1 ratio. ETV, entecavir; HBV, hepatitis B virus; LdT, telbivudine; CHB, chronic hepatitis B; ALT, alanine aminotransferase; TDF, tenofovir.
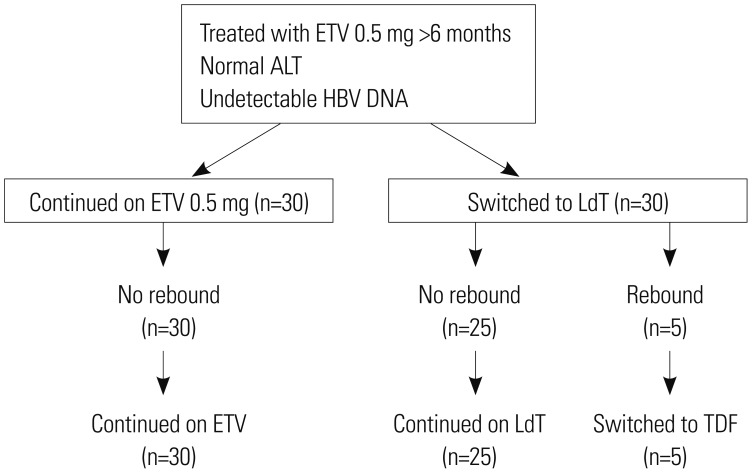
Fig. 2
Detail of five patients in entecavir-telbivudine switching group with virological breakthrough. HBV, hepatitis B virus; TDF, tenofovir.
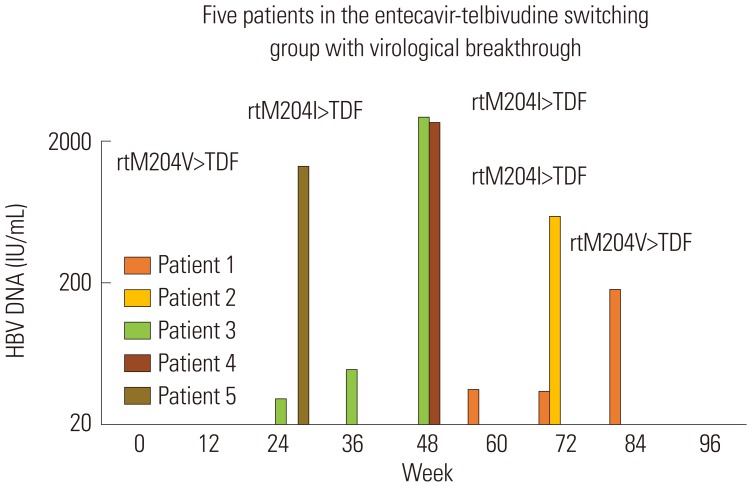
Table 1
Baseline Clinical Characteristics of Patients
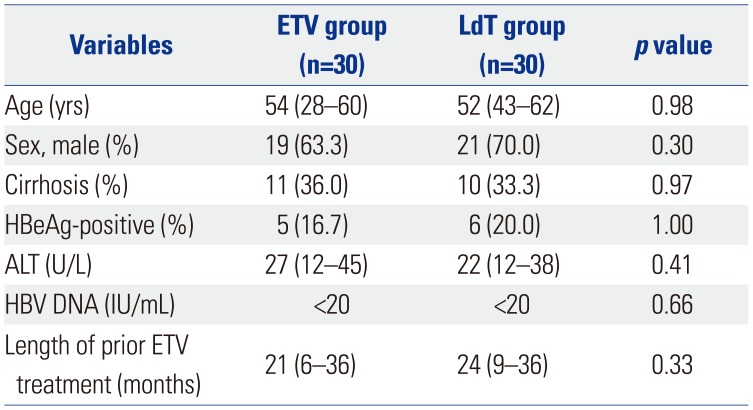
Table 2
Characteristics of Patients with Virological Rebound
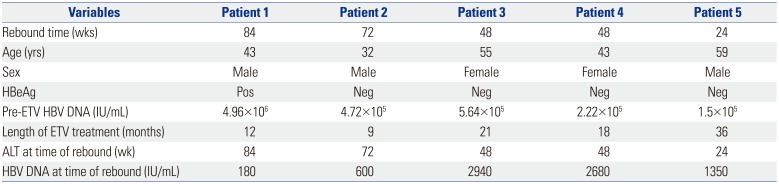
Table 3
Comparison of Patients in the ETV-Telbivudine Switching Group with and without Virological Breakthrough




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download