Abstract
In this report, the patient was pre-diagnosed as meningioma before surgery, which turned out to be meningeal melanocytoma. Hence, we will discuss the interpretation of imaging and neurological statuses that may help avoid this problem. A 45-year-old man had increasing pain around the neck 14 months prior to admission. His cervical spine MR imaging revealed a space-occupying, contrast-enhancing mass within the dura at the level of C1. The neurologic examination revealed that the patient had left-sided lower extremity weakness of 4+, decreased sensation on the right side, and hyperreflexia in both legs. Department of Neuroradiology interpreted CT and MR imaging as meningiom. The patient underwent decompression and removal of the mass. We confirmed diagnosis as meningeal melanocytoma through pathologic findings. Afterwards, we reviewed the patient's imaging work-up, which showed typical findings of meningeal melanocytoma. However, it was mistaken as meningioma, since the disease is rare.
Meningeal melanocytomas is a rare benign lesion found in the central nervous system. Although the most melanocytomas are commonly located in the posterior fossa, spinal lesions have also been reported.1 This tumor type was first reported by Limas and Tio2 in 1972, who described a tumor with histological features of both meningioma and of pigment-containing melanocytic origin. Since 1972, a number of melanocytomas have been described. However, the characteristics and natural course of this tumor type, and appropriate treatments remain poorly understood. We describe herein a case of spinal meningeal melanocytoma that was pre-diagnosed as meningioma before surgery.
A 45-year-old, previously healthy man experienced increasing pain around his neck 14 months prior to admission to the hospital. During the intervening months, his symptoms progressed and increasingly more severe; initial leg weakness became gait disturbance and later hemi-paresthesia. After approximately 12 months of symptom onset without resolution, he underwent cervical spine magnetic resonance (MR) imaging which revealed a cervical mass.
Computed tomography (CT) imaging displayed no signs of calcification or evidence of abnormal bone change, but depicted a homogenously enhancing, dura-attached mass at the C1 level. Further evaluation by MR imaging showed a contrast-enhancing mass within the dura at the level of C1, measuring about 25×19×19 mm. The mass was hyperintense on T1-weighted imaging and hypointense on T2-weighted sequences. Although the enhancement of the mass was not obvious to eyes, as the mass was hyperintense on T1-weighted imaging without enhancement, homogenous enhancement was confirmed by quantitative measures (Fig. 1). The preoperative neuro-radiological diagnosis was meningioma.
Neurologic examination revealed that the patient had a left-sided lower extremity weakness of 4, decreased sensation on the right side, and hyperreflexia in both legs. He did not exhibit any clonus or Hoffmann or Babinski reflexes. Other aspects of the examination were unremarkable.
The patient underwent decompression and removal of the mass. In order to use as baseline comparisons, motor and sensory evoked potentials were measured preoperatively. A complete laminectomy was performed in the C1 posterior arch, which revealed a black discoloration of the dura. After a dural incision at the C1 level, a black, multi-lobulated, and well-circumscribed tumor was found (Fig. 2). Gross total resection was done, and an intraoperative specimen appeared to be a melanocytic neoplasm, but the diagnosis was not conclusive.
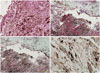
The diagnosis was confirmed by an immunohistochemical analysis (Fig. 3). Positive expression of HMB-45 and S-100 proteins was detected throughout the specimen. Mitoses, necrosis, or nuclear pleomorphism, which typically indicates a malignant melanoma, were not present in the microscopic findings. The Ki-67 labeling index was below 5%, a feature consistent with melanocytoma rather than malignant melanoma.
Postoperative clinical staging included CT scans of the chest and abdomen, whole body positron emission tomography, and a complete examination of the skin and retina. None of the exams revealed evidence of primary lesions elsewhere, suggesting the intraspinal lesion as a solitary melanoma. Postoperative cervical MR imaging was performed 4 days after the surgery and showed no evidence of residual tumor. The patient left the hospital 8 days after his surgery in a good condition. Additional follow up image was obtained 6 months after the surgery, which also showed no evidence of recurrent tumor.
The term melanocytoma was first described by Limas and Tio2 in 1972. Primary melanocytic tumors, especially of CNS origin, are extremely rare, constituting only 1% of all melanomas.3 The typical spinal patient with a meningeal melanocytoma is a female early in her fifth decade, with an insidious onset of symptoms over a period of several months to years.14 Our patient presented with a history suggestive of a radiculopathy, progressing to a myelopathy.
Histological analysis of the tumor tissue is the most accurate diagnostic tool for melanocytoma. Electron microscopy demonstrates tumor cells with large nuclei with cytoplasmic melanin pigment and prominent nucleoli.5 Mitotic activity is usually low, and necrosis is absent. By contrast, malignant melanomas present marked cellular and nuclear atypia, high mitotic activity, and necrosis. Immunohistochemical staining for the proliferation marker Ki-67 reveals a low labeling index, usually below 2%.6 Tumor cells strongly express S-100 and HMB-45 proteins, but are negative for epithelial membrane antigen, thereby excluding meningioma.7
The preoperative diagnosis of meningeal melanocytoma is usually that of meningioma due to shared characteristics on image findings and the long duration of symptoms.18 Like meningioma, melanocytoma is displayed as a solitary mass attached to the underlying dura.9
Ahluwalia, et al.1 reported that meningeal melanocytoma is usually diagnosed as meningioma. Our department of neuroradiology also interpreted CT and MR imaging as meningioma prior to surgery. After surgery, we reviewed the patient's imaging work-up. Extra-axial mass with high signal intensity on T1-weighted sequence and low signal intensity on T2-weighted sequence is a typical finding of meningeal melanocytoma. The shortening of T1 and T2 relaxation times have been attributed to the presence of paramagnetic free radicals such as indole semiquinone and semiquinonamines within the melanin.10 Radiologically, the differential diagnosis of this pigmented CNS tumor is extremely difficult, because of their similar appearance on CT and MR studies.
We emphasized differential diagnosis from meningioma. Although the incidence of meningeal melanocytoma is very low compared to meningioma, the possibility of meningeal melanocytoma should be accounted for in extra-axial mass with high signal intensity on T1-weighted sequence and low signal intensity on T2-weighted sequence and homogeneously strong enhancement.11 Meningeal melanocytoma also lacks dural tail sign, a significant hallmark of meningioma. Thus, extra-axial mass with uniform high signal intensity on T1-weighted sequence and the absence of dural tail sign can be assumed to favor meningeal melanocytoma.
Melanocytoma usually shows a benign natural course, indicating good prognosis after total excision.4 Unlike previous studies indicating a lack of benefits of adjuvant radiotherapy on meningeal melanocytoma,1 recent studies show that if a complete resection is impossible, partial resection and postoperative radiotherapy should be performed.12 Despite the high long-term survival rates, local recurrence is common.412 Therefore, routine follow-up is recommended in order to identify local tumor recurrences.
Figures and Tables
Fig. 1
An approximately 3-cm T1 high signal intensity and T2 low signal intensity homogenous enhancing intradural extramedullary mass abutting the left posterolateral aspect of the dura at C1 level, without adjacent dural thickening or dural tail sign, no evidence of abnormal bone change. (A) Contrast CT axial image. (B) Contrast CT saggital image. (C) T1-enhanced image. (D) T2 sagittal image. (E) T1 enhanced sagittal image. (F) T1 sagittal image.
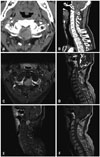
Fig. 2
(A and B) Intraoperative microscopic findings. A black colored, well-circumscribed, nodular tumor was seen. The tumor was a soft mass and well capsulated.

Fig. 3
The results of immunohistochemical staining. (A) Hematoxylin and eosin staining (×100). (B) HMB45 staining (×20). (C) S-100 protein staining (×20). (D) Ki-67 labeling index (<5%) (×100). Microscopic features supporting malignant melanoma, such as mitoses, necrosis, or nuclear pleomorphism were not observed, and the Ki-67 labeling index was low.
References
1. Ahluwalia S, Ashkan K, Casey AT. Meningeal melanocytoma: clinical features and review of the literature. Br J Neurosurg. 2003; 17:347–351.

2. Limas C, Tio FO. Meningeal melanocytoma (“melanotic meningioma”). Its melanocytic origin as revealed by electron microscopy. Cancer. 1972; 30:1286–1294.

3. Bhargava P, Grewal SS, Dewan Y, Jhawar SS, Jain V, Gupta B. et al. Craniovertebral junction melanocytoma: a case report. Turk Neurosurg. 2013; 23:539–542.
4. Clarke DB, Leblanc R, Bertrand G, Quartey GR, Snipes GJ. Meningeal melanocytoma. Report of a case and a historical comparison. J Neurosurg. 1998; 88:116–121.
5. Hou GQ, Sun JC, Zhang XJ, Shen BX, Zhu XJ, Liang L, et al. MR imaging findings of the intraspinal meningeal melanocytoma: correlation with histopathologic findings. AJNR Am J Neuroradiol. 2012; 33:1525–1529.

6. Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999; 23:745–754.
7. Liubinas SV, Maartens N, Drummond KJ. Primary melanocytic neoplasms of the central nervous system. J Clin Neurosci. 2010; 17:1227–1232.

8. Litofsky NS, Zee CS, Breeze RE, Chandrasoma PT. Meningeal melanocytoma: diagnostic criteria for a rare lesion. Neurosurgery. 1992; 31:945–948.
9. Uematsu Y, Yukawa S, Yokote H, Itakura T, Hayashi S, Komai N. Meningeal melanocytoma: magnetic resonance imaging characteristics and pathological features. Case report. J Neurosurg. 1992; 76:705–709.
10. Sealy RC. Radicals in melanin biochemistry. Methods Enzymol. 1984; 105:479–483.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download