Abstract
Percutaneous transluminal coronary angioplasty with metal stent placement has become a well-developed treatment modality for coronary stenotic lesions. Although infection involving implanted stents is rare, it can, however, occur with high morbidity and mortality. We describe herein a case of an inserted coronary stent that was infected and complicated with recurrent stent thrombosis, pseudoaneurysm formation and severe sepsis. Despite repeated intervention and bypass surgery, the patient died from severe sepsis.
Percutaneous transluminal coronary angioplasty (PTCA) with metal stent placement has become a well-developed treatment modality for coronary stenotic lesions. Although infection involving implanted stent is rare, it can, however, occur with high morbidity and mortality.1 We describe an inserted coronary stent that was infected and complicated with recurrent stent thrombosis, pseudoaneurysm formation and severe sepsis. In an effort to raise awareness of the possible fatal outcome of this situation, we herein report our experience.
A 69-year-old male with a history of hypertension, type 2 diabetes mellitus, and ischemic heart failure (ejection fraction: 32%), was admitted to our hospital to receive transurethral resection of prostate (TURP) for benign prostatic hyperplasia with prophylactic antibiotic with intravenous cefazolin. Before operation, there was no symptom of heart failure and the radiography of chest revealed no pulmonary congestion or edema. After TURP, non-ST elevation myocardial infarction with acute pulmonary edema and respiratory failure developed and, for this reason, intubation was performed. Echocardiography showed hypokinesia in inferior and anterior wall with severe left ventricle systolic dysfunction (ejection fraction: 36%), and electrocardiogram revealed sinus rhythm with premature ventricle beat and left ventricle hypertrophy. Aspirin and clopidogrel were given for myocardial infarction, diuretic agents were given for heart failure, and adequate antibiotic (Piperacillin-tazobactam) was given for superimposed pneumonia. After these therapies, there was no fever and C-reactive protein level decreased apparently, suggesting that the infection was under control, however, pulmonary edema still had poor response to diuretic. Nevertheless, because of severe cardiac dysfunction with difficulty weaning off the ventilator, coronary angiography was done 2 weeks later and revealed triple vessels disease, mainly with distal left main (LM) stenosis and chronic total occlusion of the distal right coronary artery (RCA) (Fig. 1A and B) (Supplementary Video 1, only online). Because of high surgical mortality, the patient refused coronary bypass surgery (CABG), and staged PTCA was planned instead. It was done with two drug eluting stents (DES) (Everolimus-eluting stents: Promus element 2.5×38 mm and Promus element 3.0×38 mm, Boston Scientific, Natick, MA, USA) being inserted in the distal RCA (Fig. 1C). One week later, PTCA of the left side was done, with one bare metal stent (Liberte 2.5×20 mm, Boston Scientific) being put in the middle left circumflex artery (LCX), one DES (Promus element 2.75×32 mm, Boston Scientific) in the middle left anterior coronary artery (LAD), and the other DES (Promus element 3.5×20 mm, Boston Scientific) in the LM to the proximal LAD (Fig. 1D, E, and F) (Supplementary Video 2, only online). After the PTCA, the patient's condition improved, and he was successfully weaned from the ventilator and continually received dual antiplatelet medications with aspirin and clopidogrel. However, a fever developed 4 days later. One week after the procedure, chest pain and acute ST elevation myocardial infarction developed. Emergent angiography revealed subacute stent thrombosis and pseudoaneurysm formation at the distal LM stent (Fig. 2A and B) (Supplementary Video 3, only online). Manual thrombus aspiration was performed and some pus-like debris was noted (Fig. 2C). Bacterial culture of the debris was also done. After the PTCA, the flow was restored and the intravenous glycoprotein IIb/IIIa inhibitor (tirofiban hydrochloride) and heparin (350 U/hour) were also given, accompanied with dual anti-platelet medications, but concurrent fever and persisting bacteremia were also noted. Methicillin-resistant Staphylococcus aureus (MRSA) was observed in both the blood culture and bacterial culture of pus-like debris. By the suggestion of infection specialist, antibiotic with Tigecycline (initial dose 100 mg, followed by 50 mg every 12 hours) was given. One day after the emergent PTCA, chest pain and recurrent ST elevation myocardial infarction developed again. A repeated angiography revealed recurrent stent thrombosis and progression of LM pseudoaneurysm (Fig. 2D) (Supplementary Video 4, only online). Mycotic pseudoaneurysm was diagnosed, and emergent CABG was performed with one great saphenous venous graft from aortic root to distal LAD and the other saphenous venous graft from aortic root to first obtuse marginal branch of LCX. However, the infected DES was not removed by surgeon due to high mortality risk. One day after the CABG, graft stents (GraftMaster 3.0×19 mm and 3.5×19 mm, Abbott, Santa Clara, CA, USA) were been put in the LM to the proximal LAD in case of rupture of the pseudoaneurysm (Fig. 2E to F). Despite the above aggressive therapy, intermittent fever persisted and the patient died due to severe sepsis with multiple organ failure ten days later.
In a recent review by Bosman et al. in 2014, 77 cases of metal stent infections (including coronary and peripheral stents) were reported and only 29 of them were found to have coronary stent infection.1 Despite the combined efforts of medical and surgical therapy, the mortality rate has been reported to be 38.9%.1 Therefore, infections involving an inserted stent are quite rare, nevertheless, the outcome is highly fatal. Even though stent infection is rare, we still need to pay close attention to its possibility.
The exact mechanism of stent infections remains unclear: is it a result of infection at the time of stent placement, or subsequent in stent balloon angioplasty, or of a hematogenous spread from another source of bacteremia? However, with the popular use of DES, the reports of stent infection have increased. It seems that the inhibition of neointimal growth may lead to the metallic stent remaining uncovered intraluminally, thereby forming a nidus for the adhesion of bacteria, especially in late stent infection.2 In addition to patient and stent-related risk factors, it is generally believed that risk factors of procedure–including imperfect sterility of procedure, prolonged use of indwelling catheter, and extensive changing of wires–also play an important role in early stent infection.1
More than half (57%) of PTCA cases have been reported to present with symptoms within the first month after stent placement,1 with the most common symptoms and signs being fever (93%), chills (41%), and local pain/chest pain (51.7%). Furthermore, almost 80% of image study in coronary stent infections revealed pseudoaneurysm.1 To our best knowledge, this is the first report of a PTCA patient presenting as recurrent stent thrombosis, showing that the occlusions might be caused by septic emboli or infection-related hypercoagulopathy, or both. Physicians should be aware that the stent thrombosis could be caused by stent infection, if it comes with signs of infection.
The majority of patients received emergency surgical therapy (62%) and ninety four percent (94%) of these cases also had their stents removed.1 However, the outcome was poor: the mortality in the surgery group was the lowest (38.9%), followed by the endovascular-treated group (50%) and the intravenous antibiotic only group (63.3%). The antibiotic-only therapy carries an overly high mortality. Therefore, if there are other treatment options, we recommend their combination with antibiotics. To the best of our knowledge, there have been only two case reports on endovascular therapy, and one patient succumbed to sudden death after discharge.34 In the present case of recurrent acute stent thrombosis and persistent septic shock, the cover stent or prolongation inflation might have solved the problem of perforation, perhaps even preventing further stent thrombosis. However, it could not control the progression of sepsis, because of the residual infection from the remained infected stent. Furthermore, cover stent carries risks of in-stent restenosis and possible stent thrombosis in the future. For similar cases in the future, we would suggest that surgical therapy with the removal of stent probably is the best choice. If this is not possible, however, endovascular therapy with cover stent and adequate antibiotic and anti-platelet, even anti-coagulant, may be the most reasonable second choice.
Figures and Tables
Fig. 1
Serial angiography of first time intervention. (A) Right oblique caudal view of left coronary artery shows severe stenosis of distal left main (LM, arrow) and proximal left circumflex artery. (B) Diffuse stenosis and distal total occlusion of right coronary artery (RCA). (C) Angiography of RCA after stenting. (D) Stent deployed from LM to proximal left anterior descending artery (arrow). (E) Right oblique caudal view of left coronary artery after stenting (arrow: LM stenting). (F) Spider view of left coronary artery after stenting.
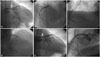
Fig. 2
(A, B, and C) Emergent angioplasty for first time stent thrombosis. (D) Emergent angioplasty for second time stent thrombosis. (E and F) Putting a graft stent. (A) Right oblique cranial view of left coronary artery shows stent thrombosis in proximal LAD (arrowheads) and pseudoaneurysm in distal LM (arrow). (B) Spider view of left coronary artery shows coronary pseudoaneurysm in distal LM (arrow). (C) Material from manual thrombus aspirator reveals thrombi with pus like material (arrow). (D) One day later, emergent angiography shows recurrent in stent thrombosis (arrowheads) and progression of coronary pseudoaneurysm in distal LM (arrow). (E) Graft stent being inserted from LM to proximal LAD (stent margin marked as arrowheads) to cover coronary pseudoaneurysm (arrow). (F) After inserting graft stent, angiography of left coronary artery reveals complete jailing of LCX. LAD, left anterior descending artery; LM, left main; LCX, left circumflex artery.
References
1. Bosman WM, Borger van der Burg BL, Schuttevaer HM, Thoma S, Hedeman Joosten PP. Infections of intravascular bare metal stents: a case report and review of literature. Eur J Vasc Endovasc Surg. 2014; 47:87–99.

2. Roubelakis A, Rawlins J, Baliulis G, Olsen S, Corbett S, Kaarne M, et al. Coronary artery rupture caused by stent infection: a rare complication. Circulation. 2015; 131:1302–1303.
3. Wu EB, Chan WW, Yu CM. Left main stem rupture caused by methicillin resistant Staphylococcus aureus infection of left main stent treated by covered stenting. Int J Cardiol. 2010; 144:e39–e41.

4. Garg RK, Sear JE, Hockstad ES. Spontaneous coronary artery perforation secondary to a sirolimus-eluting stent infection. J Invasive Cardiol. 2007; 19:E303–E306.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download