Abstract
Purpose
To identify new immunogenic HLA-A*33;03-restricted epitopes from the human papillomavirus (HPV) 16 E7 protein for immunotherapy against cervical cancer.
Materials and Methods
We synthesized fourteen overlapping 15-amino acid peptides and measured intracellular interferon-γ (IFN-γ) production in PBMC and CD8+ cytotoxic T lymphocytes (CTLs) after sensitization with these peptides using flow cytometry and ELISpot assay. The immunogenicity of epitopes was verified using a 51Cr release assay with SNU1299 cells.
Results
Among the fourteen 15-amino acid peptides, E749-63 (RAHYNIVTFCCKCDS) demonstrated the highest IFN-γ production from peripheral blood mononuclear cells (PBMCs), and CD8+ CTLs sensitized with E749-63 showed higher cytotoxic effect against SNU1299 cells than did CD8+ CTLs sensitized with other peptides or a negative control group. Thirteen 9- or 10-amino acid overlapping peptides spanning E749-63, E750-59 (AHYNIVTFCC), and E752-61 (YNIVTFCCKC) induced significantly higher IFN-γ production and cytotoxic effects against SNU1299 cells than the other peptides and negative controls, and the cytotoxicity of E750-59- and E752-61-sensitized PBMCs was induced via the cytolytic effect of CD8+ CTLs.
Conclusion
We identified E750-59 and E752-61 as novel HPV 16 E7 epitopes for HLA-A*33;03. CD8+ CTL sensitized with these peptides result in an antitumor effect against cervical cancer cells. These epitopes could be useful for immune monitoring and immunotherapy for cervical cancer and HPV 16-related diseases including anal cancer and oropharyngeal cancer.
Cervical cancer is the second most common cancer and second leading cause of cancer-related deaths in women worldwide and is highly associated with persistent high-risk types of human papillomavirus (HPV) infection.1 HPV 16 and HPV 18 are related to about 70% of invasive cervical cancer cases, and are also associated with other anogenital and oropharyngeal cancers.23 The incidence of oropharyngeal cancer is increasing rapidly, and it has been predicted that the incidence of HPV-induced oropharyngeal cancer will outstrip that of HPV-induced cervical cancer.4
Recently, two valid prophylactic vaccines for HPV have been shown to effectively prevent the incidence of HPV-associated anogenital disease in young women.5 However, only about 30% of teenagers qualified for immunization have been inoculated with the recommended dose of the vaccine;6 hence, most teenagers are not protected from HPV 16- and HPV 18-associated cancers. In addition, these prophylactic vaccines do not have a therapeutic effect on pre-existing high-risk types of HPV-infected cervical diseases.7 In spite of conventional treatments, such as radiotherapy and chemotherapy, the outcomes of treatments for recurrent or advanced-stage HPV-induced cancers are poor.8 Therefore, further research on the development of therapeutic HPV vaccines is required for HPV-associated cancers.
Previously, several reports have shown that type 1 helper T cell-dependent cellular immune responses related to cytotoxic T lymphocytes (CTLs) are crucial for the removal of pre-cancerous lesions infected with HPV 16 and HPV 18 as well as HPV-induced cancers.910 HPV 16 E7 is a nuclear protein that sustain consistent expression in cervical cancer cells, but not exist in normal cells, and have oncogenic properties.1 Thus, they are ideal tumor-specific antigen targets for cervical cancer immunotherapy, and the identification of CTL epitopes from the E7 proteins of HPV 16 and HPV 18 is fundamental for the development of therapeutic vaccines and immune monitoring systems for treated cervical cancer patients.1112
E7 is known as immunodominant HPV 16 antigens, but only a small number of CTL epitopes from E7 have been verified for HLA-A*02;01 and HLA-A*24;02 samples.1113 To establish the clinical application of a peptide-based HPV vaccine for cervical cancer, it is essential to identify immunogenic E7 epitopes restricted to each class I HLA-A allele, such as HLA-A*33;03, which is common in Asian and African countries; in approximately 20–30% of the population of Japan, China, Thailand, Pakistan, and countries of sub-Saharan Africa.14
The aim of this study was to identify new HLA-A*33;03-restricted epitopes from the HPV 16 E7 protein HLA which can be applied as therapeutic vaccines for adoptive immunotherapy using CD8+ CTLs against cervical cancer.
Peripheral blood mononuclear cells (PBMCs) were collected from healthy HLA-A*33;03 donors who were human cytomegalovirus (HCMV) seropositive. The presence of IgG and IgM HCMV antibodies in each donor was assessed using passive latex agglutination (CMVSCAN Kit; Becton-Dickinson Microbiology System, Cockeysville, MD, USA). All donors were positive for IgG and/or IgM HCMV antibodies. The major histocompatibility complex (MHC) class I genotypes were determined by sequence-specific polymerase chain reaction using genomic DNA by the HLA laboratory at the Seoul Medical Science Institute (Seoul, South Korea). PBMCs were isolated from peripheral blood of donors by density gradient centrifugation using Ficoll-Hypaque 1.077 (Pharmacia Biotech, Wilkstrom, Sweden). The isolated mononuclear cells were washed twice with phosphate-buffered saline (PBS; Gibco, Grand Island, NY, USA) and cryopreserved at -160℃ in human AB+ serum containing 10% dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA). This research was approved by the Institutional Review Board of Yonsei University Health System, and all participants provided written informed consent.
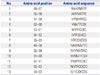
A total of fourteen 15-amino acids peptides that overlap by 8 residues for the HPV 16 E7 protein were synthesized (A & Pep, Chungwon-gun, South Korea) (Table 1). After determining the candidate immunodominant 15-amino acid peptides through screening and the verification test described below, 9- or 10-amino acid peptides spanning the candidate 15-amino acid peptides were synthesized (Table 2). The purity of the synthetic peptides was confirmed to be greater than or equal to 95% by high-performance liquid chromatography. The peptides were diluted in 1% DMSO containing DEPC-treated water (Invitrogen, Carlsbad, CA, USA) to working solution concentrations (1 µg/µL) and stored at -80℃ until used.
Peptide-loaded autologous dendritic cells (DCs) were generated as previously described, with minor modifications.15 PBMCs were incubated at 37℃ for 2 hours in complete RPMI medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Gibco) and 1% antibiotics (Invitrogen). Adherent monocytes were resuspended at a concentration of 5×106 cells/mL in complete RPMI medium supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) (1500 IU/mL; PeproTech, Rocky Hill, NJ, USA) and interleukin (IL)-4 (1200 IU/mL; PeproTech). On days 2, 4, and 6 of culture, fresh cytokines were added. On day 5 of culture, 10 ng/mL of tumor necrosis factor-α (TNF-α) (R&D Systems, Minneapolis, MN, USA) was added to induce maturation of DCs. After maturation, autologous DCs were stimulated with peptides for 6 hours. PBMCs were plated at a concentration of 2×106 cells per well in a 24-well plate (Nunc, Rochester, NY, USA) with 2 mL of complete RPMI medium. PBMCs were sensitized with synthetic HPV 16 E7 peptides (10 µg/mL/well), and 1000 IU/mL/well of recombinant human IL-2 (rhIL-2; PeproTech) was added. Additionally, rhIL-2 (1000 IU/mL/well) was added to the culture every other day. After a 1-week expansion period, peptide-pulsed autologous DCs (4-10×106/well) were added to the PBMCs on day 7, incubated for 6 hours, and analyzed by flow cytometry. DC-treated PBMCs were cultured for additional 7 days, and used for cytotoxicity assays. CD8-positive and -negative cells were isolated from 2-week-sensitized PBMCs for the cytotoxicity assay using Dynabeads® FlowComp™ Human CD8 Kit (Invitrogen).
PerCP-conjugated anti-CD3, PE-conjugated anti-CD8, FITC-conjugated anti-intracellular interferon-γ (IFN-γ), and PerCP-conjugated anti-CD69 were purchased from BD Biosciences (San Jose, CA, USA). For each sample, 75000 events were acquired in the FSC/SSC lymphocyte gate using a FACSCalibur flow cytometer (BD Biosciences). For data analysis using CELLQuest software (BD Biosciences), CD8+IFN-γ+ cells were expressed as the percentage of the respective reference population. The evaluation of responses was previously described in detail.16 Briefly, peptide-sensitized PBMCs (1×106 cell/mL) stimulated with phytohemagglutinin (PHA; Sigma, St. Louis, MO, USA) and PBMCs stimulated with autologous DCs loaded with none of the peptides were used for the positive and negative control groups, respectively. PBMCs stimulated with autologous DCs loaded with CMV pp65495–503 (NLVPMVATV, HLA-A*02;01) or CMV pp6591-100 (SVNVHNPTGR, HLA-A* 33;03) were used for the positive or negative control according to the HLA type of donors. INF-γ produced in CD3+CD8+ CTLs was calculated as previously described.11 After one-hour stimulation, 10 µg of brefeldin A (Sigma, St. Louis, MO, USA) was added to each well. After an additional 5 hours of incubation, PBMCs were washed once with PBS and were then incubated in PBS containing 1 mM EDTA for 10 minutes. After two washes with PBS containing 5% FBS, the cells were incubated with fluorescence-conjugated monoclonal anti-CD3+, anti-CD8+, anti-INF-γ+, and anti-CD69 antibodies for 15 minutes on ice in the dark.
IFN-γ production was determined in CD8+ T cell stimulated with HPV 16 E7 peptides. After a 1-week expansion period, peptide-pulsed autologous DCs (4-10×106/well) were added to the PBMCs on day 7, incubated overnight. After incubation, CD8+ T cells were isolated from PBMCs using Dynabeads® FlowComp™ Human CD8 Kit (Invitrogen). Then, 3×105 cells/well were plated in triplicate for each treatment in the Enzyme linked immunospot (ELISpot) plates (Millipore, Billerica, MA, USA). Plates were previously coated with anti-IFN-γ antibody (Mabtech, Nacka Strand, Sweden) at 4℃ overnight. CD8+ T cells were incubated with HPV 16 E7 peptides at 37℃ and 5% CO2. PHA was added at 10 µg/mL as a positive control, with RPMI only as a negative control. After 24 hours incubation, 100 µL of Biotinylated IFN-γ detection antibody diluted in 1:2000 in PBS was added to each well (Biolegend, San Diego, CA, USA) and incubated at room temperature (RT) for 1.5 hours. After washing the plate 6 times with PBS, 100 µL of streptavidin-alkaline phosphatase (Invitrogen) diluted 1:1000 in PBS was added. The plate was incubated at RT for 1 hour in the dark and developed with color solution by mixing 10 mL of Tris-MgCl2 buffer with 100 µL of NBT solution (Bio-Rad, Berkeley, CA, USA) and 100 µL of BCIP solution (Bio-Rad). The plate was washed 6 times with water and dried before reading. The spots were quantified with an ELISpot plate reader and software version 3.5 (AID ELISpot Reader System, Strassberg, Germany). Data were obtained by calculating the means of triplicate wells. ELISpot data were represented as the total IFN-γ spot-forming units (SFU)/3×105 CD8+ T cells. PBMCs stimulated with autologous DCs loaded with CMV pp65495-503 (NLVPMVATV, HLA-A*02;01) or CMV pp6591-100 (SVNVHNPTGR, HLA-A*33;03) were used for the positive or negative control according to the HLA type of donors.
The human cervical cancer cell line SNU1299 (HLA-A*02;01/33;03) was purchased from the Korean Cell Line Bank (Seoul, South Korea), and was tested and authenticated. The SNU1299 cell line was expanded and frozen in aliquots within 4 weeks of purchase. SNU1299 cells were thawed and cultured at 37℃ and 5% CO2 in complete RPMI medium for no more than 8 passages. Immortalized Epstein-Barr virus-B lymphoblastoid cell (EBV-BLC) lines (HLA-A*33;03/33;03 or HLA-A*02;01/02;01) and CMV infected-fibroblast (HLA-A*33;03/33;03) were cultured at 37℃ and 5% CO2 in complete RPMI medium. Cells were routinely tested for the absence of mycoplasma.
A cytotoxicity assay was performed employing the 51Cr release assay as previously described.11 Briefly, SNU1299 cells were labeled for 45 min with 51Cr (100 mCi/106 cells, Perkin Elmer, Waltham, MA, USA), and then washed in PBS, and dispensed into U-bottom 96-well plates in triplicate (Nunc) at 4×103 cells/well. Peptide-pulsed PBMCs were added at an effector:target ratio of either 10:1, 30:1, 50:1, or 100:1 and CD8+ T cells were added at an effector:target ratio of either 1:1, 3:1, 5:1, or 10:1. The cells were incubated for 6 hours, and the supernatant was analyzed using a WIZARD2 Automatic Gamma Counter (Perkin Elmer, Waltham, MA, USA). The spontaneous and total release for each target were used to calculate the percentage of specific release according to the following formula: % specific release=(experimental counts per minute-spontaneous counts per minute)/(total counts per minute-spontaneous counts per minute)×100. For the effectors, PBMCs sensitized with autologous DCs loaded with CMV pp65495-503 (NLVPMVATV, HLA-A*02;01) or CMV pp6591-100 (SVNVHNPTGR, HLA-A*33;03) were used for the positive or negative control according to the HLA type of donors. CMV specific peptide loaded-EBV-BLC or CMV infected-fibroblast were used as target cells.
Data presented as means±standard errors (SEs) were representative of at least three independent experiments. The Mann-Whitney U test was used for non-parametric comparisons of two variables. For all statistical analyses, the social sciences software package SPSS (version 13.0; SPSS Inc., Chicago, IL, USA) was used and a p value of less than 0.05 was considered statistically significant.
We first determined which of the fourteen HPV 16 E7 peptides have immunogenic potency for the generation of CTLs. Thus, PBMCs from HLA-A*33;03 donors were sensitized with each of the candidate 15-amino acid peptides for one week, and IFN-γ production of PBMCs was measured. Among the fourteen 15-amino acid peptides, HPV 16 E749-63 (RAHYNIVTFCCKCDS) induced significantly greater IFN-γ production of PBMCs than the other peptides as well as the negative control (p=0.037) (Fig. 1A and B). Here, PBMCs from HLA-A*33;03 donors stimulated with autologous DCs loaded with CMV pp65495-503 (NLVPMVATV, HLA-A*02;01) and with no peptide were used for the negative controls, whereas PBMCs from HLA-A*33;03 donors stimulated with autologous DCs loaded with CMV pp6591-100 (SVNVHNPTGR, HLA-A*33;03) and PHA were used for the positive controls.
To provide further evidence, ELISpot assays were performed and IFN-γ+ spot forming numbers were counted after in vitro sensitization of CD8+ CTLs with each peptide. As shown in Fig. 1C, E749-63 produced significantly higher numbers of IFN-γ+ spots than other candidate peptides and negative control (p=0.002). These results imply that E749-63 peptide is potential HLA-A* 33;03-restricted HPV 16 E7 epitopes: PBMCs from HLA-A* 33;03 donors stimulated with autologous DCs loaded with CMV pp65495-503 (NLVPMVATV, HLA-A*02;01) and with no peptide were used for the negative controls, whereas PBMCs from HLA-A*33;03 donors stimulated with autologous DCs loaded with CMV pp6591-100 (SVNVHNPTGR, HLA-A*33;03) and with PHA were used for the positive controls.
To confirm the immunogenicity of selected E749-63 peptide by a flow cytometry analysis and ELISpot assay, HLA-A*33;03-restricted cytotoxicity was measured by 51Cr release. E749-63 peptide-sensitized PBMCs from HLA-A*33;03 donors were used for two weeks as the effector cells for the 51Cr release assay against HLA-A-matched SNU1299 cells. As shown in Fig. 1D, PBMCs sensitized with E749-63 showed significantly higher 51Cr release than the cells in the negative control group, suggesting that E749-63 is a potential HLA-A*3303-restricted HPV 16 E7 epitope. For the positive control, PBMCs stimulated with autologous DCs loaded with CMV pp6591-100 (SVNVHNPTGR, HLA-A* 33;03) as effectors and CMV infected-fibroblasts (HLA-A* 33;03) were used as the target cells. For the negative control, PBMCs stimulated with autologous DCs loaded with CMV pp65495-503 (NLVPMVATV, HLA-A*02;01) as effectors and CMV infected-fibroblasts (HLA-A*33;03) were used as the target cells.
We synthesized a total of thirteen overlapping 9- or 10-amino acid peptides spanning E749-63, and used them to determine precise HLA-A*33;03-restricted HPV 16 E7 epitopes. After two weeks of in vitro sensitization with each of the thirteen candidate peptides, intracellular IFN-γ production in PBMCs from HLA-A*33;03 donors was assessed using flow cytometry. Among these peptides, E750-59 (AHYNIVTFCC)- and E752-61 (YNIVTFCCKC)-sensitized PBMCs showed higher IFN-γ production than other peptides and negative controls (p=0.036, 0.047, respectively) (Fig. 2A and B). PBMCs from HLA-A*33;03 donors stimulated with autologous DCs loaded with CMV pp65495-503 (NLVPMVATV, HLA-A*02;01) and with no peptide were used as negative controls, while PBMCs from HLA-A*33;03 donors stimulated with autologous DCs loaded with CMV pp6591-100 (SVNVHNPTGR, HLA-A*33;03) and with PHA as positive controls.
To verify HLA-A*33;03-restricted cytotoxicity induced by E761-69 and E767-76, a 51Cr release assay against SNU1299 cells was performed using PBMCs from HLA-A*33;03 donors sensitized with E750-59 and E752-61. The results showed that E750-59- and E752-61-sensitized PBMCs demonstrated significantly higher cytotoxicity against SNU1299 cells than the negative control group (Fig. 2C). For the positive control, PBMCs stimulated with autologous DCs loaded with CMV pp6591-100 (SVNVHNPTGR, HLA-A*33;03) were used as effectors and CMV infected-fibroblasts (HLA-A*33;03) were used as the target cells. For the negative control, PBMCs stimulated with autologous DCs loaded with CMV pp65495-503 (NLVPMVATV, HLA-A*02;01) were used as effectors and CMV infected-fibroblasts (HLA-A*33;03) were used as the target cells. PBMCs stimulated with autologous DCs loaded with no peptide as effectors and SNU1299 cells as target cells were used for another negative control.
To further assess the epitope-specificity of the cytotoxic effect by these peptide-sensitized PBMCs, we performed a 51Cr release assay using EBV-BLCs expressing HLA-A*33;03 as target cells. PBMCs from HLA-A*33;03 donors sensitized with E750-59 and E752-61 showed cytolytic effects against EBV-BLCs loaded with the same peptide, but not against EBV-BLCs loaded with none of peptides (the negative controls) (Fig. 2D). PBMCs from HLA-A*33;03 donors sensitized with CMV pp6591-100 (SVNVHNPTGR, HLA-A*33;03) and EBV-BLCs loaded with the same peptide were used as the positive control.
In addition, higher CD69 expression on CD8+INF-γ+ CTLs was consistently induced by sensitization of E750-59 and E752-61 peptides than for the negative control group (Fig. 2E).
We further examined whether the cytotoxicity of peptide-sensitized PBMCs was related to cytolytic activity of CD8+ CTLs in PBMCs. Thus, 51Cr release assays were performed using isolated CD8+ CTLs and CD8+ T lymphocyte-depleted PBMCs against SNU1299 cells. E749-63-sensitized CD8+ CTLs showed significantly higher cytolytic effects against SNU1299 cells than the CD8+ T lymphocyte-depleted PBMCs as well as the negative control group (PBMCS stimulated with no peptide) (Fig. 3A). Additionally, when we performed a cytotoxicity assay using E750-59 and E752-61-sensitized CD8+ CTLs, the cytotoxicity against SNU1299 cells was higher than that of CD8+ T lymphocyte-depleted PBMCs as well as the negative control group (PBMCS stimulated with no peptide) (Fig. 3B).
Currently, approximately 500000 newly diagnosed cases of cervical cancer with an approximately 50% mortality rate are reported each year worldwide.17 Since conventional treatments against early-stage, late-stage, or recurrent cervical cancer are associated with a high probability of recurrence,1819 there is still a need to develop innovative immune therapeutic methods that can be combined with current treatment to further improve the survival rate of patients with cervical cancer.
Previous studies have shown that HPV 16-specific CD8+ T cell responses play a critical role in the regression of HPV 16-infected precancerous lesions and also in the clearance of HPV 16-induced cervical cancer.2021 Because the HPV E6 and E7 proteins are constitutively expressed in both HPV-infected cells and cervical cancer cells, these oncoproteins are potential targets for immunotherapy against HPV-associated disease. Therefore, the identification of immunodominant epitopes from HPV E6 and E7 for cytotoxic CD8+ T cells is indispensable for CTL-based immunotherapy in cervical cancer. Although HLA-A33 is a common HLA-A molecule expressed in humans, HLA-A33-restricted HPV 16 E7 epitopes have not yet been identified. Here, we identified new HLA-A*33;03-restricted HPV 16 E7 specific epitopes using 15-amino acid peptides overlapped by 8 residues covering the full-length HPV 16 E7 protein.
In the present study, 15-amino acid peptides were synthesized and flow cytometry and ELISpot assay were used to measure INF-γ production in PBMCs from HLA-A*33;03 donors. Among the fourteen 15-amino acids peptides, HPV E749-63 induced significantly higher IFN-γ production in PBMCs than other peptides, and the 51Cr releasing assay showed that these peptides induced a cytotoxic effect against SNU1299 cells (Fig. 1). The virally-infected cancer cells can be recognized by CD8+ T cells through immunogenic protein fragments of 8 to 12 amino acids generated by extracellular antigen processing on the cell surface with HLA class I molecules.22 Therefore, we synthesized six 9-amino acid peptides with 8 overlapping residues and seven 10-amino acid peptides with 9 overlapping residues spanning E746-93, and established precise HLA-A*33;03-restricted HPV 16 E7 epitopes. Consequentially, two of the thirteen peptides induced significantly higher IFN-γ production in PBMCs (Fig. 2A). Additionally, we found that the two selected E7 peptides were present in the HLA-A*33;03 molecules of SNU1299 cells and they strongly induced cytotoxicity against SNU1299 cells (Fig. 2C) similar to selected E7 peptide-pulsed EBV-BLCs (Fig. 2D). These results indicate that they are naturally processed and present in human cervical cancer cells, and that peptide-sensitized CTL recognized and lysed E750-59- and E752-61-loaded cervical cancer cells. Furthermore, we investigated whether peptide-specific cytotoxicity was induced by the cytotoxic effect of CD8+ CTLs included in PBMCs, and found that the E750-59- and E752-61-sensitized and isolated CD8+ CTLs from PBMCs demonstrated significantly greater cytotoxicity than CD8-depleted PBMCs against SUN1299 cells (Fig. 3), thus confirming that the cytolytic effect of CD8+ CTLs is critical for peptide-based immunotherapy in cervical cancer.
Some limitation might be found in this study. A previous study reported that the specificity of tumor cell lysis by peptide-induced CTL should be interpreted with caution, because CTL reactivity against cervical cancer cell lines could possibly be due to cross-reactivity on allogeneic HLA molecules rather than to E7 recognition.23 Others reported also that this type of vaccine therapy may ultimately be better suited for the treatment of patients with pre-invasive disease, because less advanced lesions such as high-grade dysplasia or carcinoma in situ are generally much smaller lesions that are more stable genetically.24 However, more recent studies showed many therapeutic HPV vaccines in clinical trials with promising results. In these studies, adoptive T-cell therapy showed clinical activity in a phase II trial involving advanced cervical cancer patients. Several trials, involving peptide-protein-based vaccines and live-vector based vaccines, also demonstrated that these approaches are effective in cervical intraepithelial neoplasia (CIN) as well as in advanced cervical cancer (CC) patients. HPV therapeutic vaccines must be regarded as a therapeutic option in cervical disease.25 The strength of the present study is the fact that identified HPV 16 E7 specific peptides could play an important role as a basic platform for both the development of peptide-induced CTL vaccines and development of peptide-based CTL immune monitoring system which are very specific for HLA-A33 cervical cancer patients in Korean and Asian peoples. Furthermore, identified HPV 16 E7 specific peptides in this study could be used for combination of HPV therapeutic vaccines with radiotherapy, chemotherapy, immunomodulators or immune checkpoint inhibitors, and would open a new and interesting scenario for HPV 16 E7 positive cancers including cervix cancer and oropharyngeal cancer in near future.
In summary, we identified novel HPV 16 E7 epitopes E761-69 and E767-76 for the HLA-A*33;03 allele and showed that E761-69- and E767-76-sensitized PBMC as well as CD8+ CTLs have an antitumor cytotoxic effect against cervical cancer cells. These epitopes might be helpful for immunotherapy and immune monitoring for cervical cancer as well as other HPV 16-assocated diseases.
Figures and Tables
 | Fig. 1Quantitation of IFN-γ production and a cytotoxicity assay of candidate 15-amino acid peptide-sensitized CTLs. (A) Flow cytometry analyses were performed to measure intracellular IFN-γ production of PBMCs sensitized with candidate peptides. E749-63 induced significantly higher IFN-γ production in PBMCs from HLA-A*33;03 subjects than negative controls (non-peptide). The data are representative of ten independent experiments using PBMCs from HLA-A*33;03 subjects. (B) The bar graph depicts the numbers of each peptide-specific INF-γ+CD8+ T cells per 7.5×104 PBMCs. Columns, mean (n=10); bars, SE. (C) ELISpot assays were performed to measure IFN-γ production from CD8+ CTL that were sensitized with fourteen candidate peptides. HPV 16 E749-63 induced greater number of IFN-γ+ spots from CD8+ CTLs of HLA-A*33;03 donors than other peptides as well as negative control. The bar graph depicts the numbers of SFU of INF-γ produced by CD8+ T cells. Columns, mean (n=6); bars, SE. (D) E749-63 (▵)-sensitized PBMCs lysed a significantly higher number of SNU1299 cells than negative control in a cytotoxic assay using 51Cr release. Point, mean (n=10); bars, SE. CMV A33 (○)-sensitized PBMCs was used as a positive control and non-peptide (•) or CMV A02 (▾)-sensitized PBMCs were used as negative controls matched with each donor's HLA type. All statistically significant differences between the test group and the control group (non-peptide) were determined using the Mann-Whitney U test. *p<0.05, **p<0.01. IFN-γ, interferon-γ; CTLs, cytotoxic T lymphocytes; PBMCs, peripheral blood mononuclear cells; SE, standard error; HPV, human papillomavirus. |
 | Fig. 2Quantitation of intracellular IFN-γ production and a cytotoxicity assay of candidate 9- or 10-amino acid peptide-sensitized CTLs. (A) E750-59 and E752-61 induced significantly higher IFN-γ production in PBMCs from HLA-A*33;03 subjects than negative controls (non-peptide). The data are representative of ten independent experiments using PBMCs from HLA-A*33;03 subjects. (B) The bar graph depicts the number of each peptide-specific INF-γ+CD8+ T cells per 7.5×104 PBMCs. Columns, mean (n=10); bars, SE. Statistically significant differences between the test group and the non-peptide group were determined using the Mann-Whitney U test. *p<0.05, **p<0.01. (C) E750-59 (▵)- and E752-61 (▪)-sensitized PBMC lysed a significantly higher number of SNU129 cells than negative control (non-peptide, •). CMV A33 (○)-sensitized PBMCs was used as a positive control and CMV A02 (▾)-sensitized PBMCs was used as a negative control. Point, mean (n=10); bars, SE. (D) E750-59 (▾)- and E752-61 (▵)-sensitized PBMCs lysed a significantly higher number of EBV-BLCs than negative control. CMV A33 (○)-sensitized PBMCs was used as a positive control and non-peptide sensitized-PBMCs (none, •) or non-peptide pulsed-EBV-BLCs (▪, □) were used as negative controls. Point, mean (n=10); bars, SE. (E) CD69 expression by CD8+ T cells was measured by flow cytometry to determine the activation of CD8+ CTLs after a 1-week sensitization with each candidate peptide, and E750-59 and E752-61 -sensitized CD8+ CTLs expressed higher quantities of CD69 than negative control. CMV A33-sensitized CD8+ CTLs were used as a positive control and non-peptide or CMV A02-sensitized CD8+ CTLs were used as negative controls. Data are representative of four independent experiments using PBMCs from HLA-A*3303 subjects. IFN-γ, interferon-γ; CTLs, cytotoxic T lymphocytes; PBMCs, peripheral blood mononuclear cells; SE, standard error; EBV-BLCs, Epstein-Barr virus-B lymphoblastoid cells. |
 | Fig. 3HPV 16 E7 peptide-specific cytotoxicity by the cytolytic effect of CD8+ CTLs in PBMCs (A) E749-63-sensitized- and isolated-CD8+ CTLs (▪) as well as E749-63-sensitized PBMCs (○) lysed significantly greater quantities of SNU1299 cells than the CD8+ T lymphocyte-depleted PBMCs (▵) and non-peptide sensitized-PBMCs (•) used as a negative control. Point, mean (n=7); bar, SE. (B) E750-59 (▾)- and E752-61 (▵)-sensitized and isolated-CD8+ CTLs also showed significantly higher cytotoxicity against SNU1299 cells than CD8+ T lymphocyte-depleted PBMCs (▪, □) as well as the negative control (non-peptide, •). Point, mean (n=7); bar, SE. HPV, human papillomavirus; PBMCs, peripheral blood mononuclear cells; SE, standard error; CTLs, cytotoxic T lymphocytes. |
Table 1
List of 15-Amino Acid Overlapping Peptides Spanning HPV 16 E7 Protein
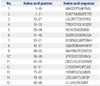
Table 2
List of 9- or 10-Amino Acid Overlapping Peptides Spanning E749-63
References
1. zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002; 2:342–350.

2. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003; 348:518–527.

3. Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002; 55:244–265.

4. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011; 29:4294–4301.

5. Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010; 10:845–852.

6. Centers for Disease Control and Prevention. National survey shows HPV vaccine rates trail other teen vaccines. 2011. accessed on 2011 August 25. Available at: https://www.cdc.gov/media/releases/2011/p0825_hpv_vaccine.htm.
7. Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007; 298:743–753.

9. Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, et al. Immunotherapy for cervical cancer: research status and clinical potential. BioDrugs. 2010; 24:109–129.
10. van der Burg SH, Melief CJ. Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol. 2011; 23:252–257.

11. Jang S, Kim YT, Chung HW, Lee KR, Lim JB, Lee K. Identification of novel immunogenic human leukocyte antigen-A 2402-binding epitopes of human papillomavirus type 16 E7 for immunotherapy against human cervical cancer. Cancer. 2012; 118:2173–2183.

12. Yan J, Harris K, Khan AS, Draghia-Akli R, Sewell D, Weiner DB. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine. 2008; 26:5210–5215.

13. Ressing ME, Sette A, Brandt RM, Ruppert J, Wentworth PA, Hartman M, et al. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol. 1995; 154:5934–5943.
14. Clayton J, Lonjou C. Allele and Haplotype frequencies for HLA loci in various ethnic groups. In : Charron D, editor. Genetic diversity of HLA. Functional and medical implications. Paris: EDK;1997. p. 665–820.
15. Lim JB, Kim HO, Jeong SH, Ha JE, Jang S, Lee SG, et al. Identification of HLA-A*2402-restricted HCMV immediate early-1 (IE-1) epitopes as targets for CD8+ HCMV-specific cytotoxic T lymphocytes. J Transl Med. 2009; 7:72.

16. Rauser G, Einsele H, Sinzger C, Wernet D, Kuntz G, Assenmacher M, et al. Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood. 2004; 103:3565–3572.

17. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

18. Schorge JO, Molpus KL, Koelliker D, Nikrui N, Goodman A, Fuller AF Jr. Stage IB and IIA cervical cancer with negative lymph nodes: the role of adjuvant radiotherapy after radical hysterectomy. Gynecol Oncol. 1997; 66:31–35.

19. Bonomi P, Blessing J, Ball H, Hanjani P, DiSaia PJ. A phase II evaluation of cisplatin and 5-fluorouracil in patients with advanced squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989; 34:357–359.

20. Øvestad IT, Gudlaugsson E, Skaland I, Malpica A, Kruse AJ, Janssen EA, et al. Local immune response in the microenvironment of CIN2-3 with and without spontaneous regression. Mod Pathol. 2010; 23:1231–1240.

21. Jung AC, Guihard S, Krugell S, Ledrappier S, Brochot A, Dalstein V, et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer. 2013; 132:E26–E36.

22. Kubo RT, Sette A, Grey HM, Appella E, Sakaguchi K, Zhu NZ, et al. Definition of specific peptide motifs for four major HLA-A alleles. J Immunol. 1994; 152:3913–3924.
23. Jochmus I, Osen W, Altmann A, Buck G, Hofmann B, Schneider A, et al. Specificity of human cytotoxic T lymphocytes induced by a human papillomavirus type 16 E7-derived peptide. J Gen Virol. 1997; 78(Pt 7):1689–1695.

24. Steller MA, Gurski KJ, Murakami M, Daniel RW, Shah KV, Celis E, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res. 1998; 4:2103–2109.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download