Abstract
Purpose
Transforming growth factor-β-induced protein (TGFBIp) is highly expressed in the cornea, and mutant TGFBIp induces corneal diseases. However, the function of TGFBIp in cornea epithelium is not fully investigated. Here, we tested the importance of TGFBIp in regulation of gene expression and corneal epithelial cell (CEC) activity.
Materials and Methods
The effect of TGFBIp on CEC activity was analyzed by cell migration, adhesion, proliferation and wound healing assay. Analysis of gene expression was examined by western blot and quantitative reverse transcription PCR.
Results
The results demonstrated that TGFBIp increased adhesion, migration, proliferation, and wound healing of CECs. Analysis of gene expression presented that TGFBIp-stimulated CECs exhibited increased expression of mucin family genes, such as MUC1, -4, -5AC, and -16. Furthermore, TGFBIp treatment increased the expression of MUC1, -4, -5AC, -7, and -16 in conjunctival epithelial cells. TGFBIp also increased the activity of intracellular signaling molecules ERK and AKT in CECs. Using pharmacologic inhibitors of ERK and AKT, we showed that the expression of mucin genes by TGFBIp is mediated by the activation of ERK and AKT signaling.
Conclusion
Our findings demonstrate that the locally generated TGFBIp in the cornea may contribute to wound healing of CECs by enhancing the migration, adhesion, and proliferation of CECs. In addition, our results suggest that TGFBIp has a protective effect on ocular surfaces by inducing the expression of mucin genes in corneal and conjunctival epithelial cells. These data suggest that TGFBIp is a useful therapeutic target for patients with corneal wounds.
The cornea and conjunctiva, which make up the ocular surface, are the tissues to interact with the external environment of the eye. The tear film of ocular surface is composed of multiple layers induced by tissues and glands.1 Each layer contains specific molecules to protect surface cells from the environment.234 A major protective mechanism is secretion of the innermost layer of the tear film known as the mucous layer.2 Both the cornea and conjunctiva epithelial cells generate insoluble mucins, whereas the conjunctiva epithelial cells produce soluble mucins.345 The mucins in the tear film maintain hydration and provide lubrication between the cells of the ocular surface and conjunctiva during blinking,67 Additionally, mucins prevent pathogens from binding to the ocular surface.8
Mucin production is regulated at multiple levels, including transcription, translation, post-translational modification, and cellular differentiation.91011 Expression of membrane-spanning insoluble mucin is dependent upon the cell differentiation st-age. Therefore, mucin production is dependent on the factors that regulate differentiation.12 In the cornea, MUC1, -4, and -16 are presented on the apical cells and mucin mRNA and protein have been shown to be upregulated by serum.13 Fibroblast growth factor 10 increased MUC1 and MUC4,14 and retinoic acid upregulated the protein expression of MUC16 in a conjunctival epithelial cell line.15 In addition, dexamethasone increased MUC16 expression in the corneal epithelial cells (CECs).16 Expression of MUC7, a small secretory mucin, is induced by lipopolysaccharide interleukin (IL)-1β, IL-4, IL-13, tumor necrosis factor alpha, and epidermal growth factor (EGF) in airway epithelial cells.17 However, the regulation of MUC7 production in ocular tissues is limited. Expression of MUC5AC, a gel-forming mucin, is shown to be upregulated in the presence of retinoic acid,18 but little is known about the gene expression of gel-forming mucins in the eye.
Transforming growth factor-β-induced protein (TGFBIp) is an extracellular matrix (ECM) protein and is expressed in various types of cells, including smooth muscle cells, chondrocytes, fibroblasts, tumor cells, and CECs.1920212223 As a component of the ECM, TGFBIp interacts with ECM proteins, such as laminin, collagen, fibronectin, and glycosaminoglycans,19 and increases cell migration, differentiation, adhesion, wound healing, metastasis, spread, and proliferation through interactions with intergrins.242526272829
In cornea, mutation of TGFBI gene is responsible for 5q31-linked autosomal dominant corneal dystrophies.30 These diseases are characterized by accumulation of deposits in the cornea, resulting in a loss of transparency. Corneal dystrophy is characterized by a reduction in visual acuity and often culminates in blindness due to the accumulation of protein deposits in the cornea. Immunohistological studies demonstrated that mutant TGFBIp is abundant in the pathologic deposits in all TGFBIp-related corneal dystrophies,31 while wild-type TGFBIp exists mainly in the extracellular space of CECs, below the corneal epithelial layer, and in the corneal stromal layer.31 TGFBIp appears to exist in both a covalently bound state and a free soluble form.32 The bound state TGFBIp may exhibit as anchors for cells in the ECM, while the soluble TGFBIp may serve a regulatory function. An sodium dodecyl sulfate (SDS)-insoluble fraction of TGFBIp is covalently bound to the type XII collagen, and its interaction provide anchoring for cells to the ECM.32 Therefore, interaction between TGFBIp and collagen is important for understanding the homeostasis of cornea and the pathobiology of TGFBI-linked corneal dystrophies. However, the role of wild-type TGFBIp in corneal/conjunctival epithelial cells is largely unknown, despite its clear expression in the cornea.
Therefore, the identification of the novel role of TGFBIp in healthy corneal/conjunctival epithelial cells is important for the understanding of the cornea pathophysiology. Here, we report a novel role and underlying signaling mechanism for the TGFBIp in CEC and mucin expression.
Immortalized SV40 human CEC line (p4) was obtained from Dr. Hitoshi Watanabe (Osaka University, Osaka, Japan).33 Cells were cultured in Dulbeco's modified ragle's media (DMEM)/F12 (Invitrogen, Carlsbad, CA, USA) supplemented with human recombinant EGF (10 ng/mL) (Upstate Biotechnology, Lake Placid, NY, USA) and 10% fetal bovine serum. Cells were incubated in a CO2-regulated incubator and the medium was renewed every two days.
Human conjunctival epithelial cells were given as a gift by Dr. Tae-im Kim.34 Human conjunctival tissues were donated by the YONSEI eye bank (Severance EYE & ENT Hospital, Seoul, Korea). Conjunctiva was dissected 2-mm lateral to the limbus of the cornea and incubated with 1.4 U dispase (Sigma-Aldrich Corp., St. Louis, MO, USA) for 1 hour in a 37℃ incubator. Conjunctival epithelial cells were then removed with a cell scraper and seeded on 60 mm plate. Cells were cultured in KGM-Gold medium (Lonza, Walkersville, MD, USA). The culture medium was changed every other day until the cells reached 60% to 70% confluence (~5–6 days). After reaching confluence, epithelial cells (1×105 cells) were used to analyze gene expression.
Human corneal tissue was harvested from healthy corneas from the eye bank after penetrating or lamellar keratoplasty. Donor confidentiality was maintained in accordance with the Declaration of Helsinki and was approved by the Severance Hospital IRB Committee (CR04124), Yonsei University.
Corneas (described below) and epithelial tissue were collected by scraping and then stored at -80℃ until RNA extraction and analysis of mucin expression by quantitative reverse transcription PCR (qRT-PCR).
Primary CECs were isolated from corneas using a method described by Maldonado and Furcht.35 Briefly, the cornea was placed in a 35 mm plate containing dispase II (1.2 U/mL) and incubated for 3-hour at 37℃. The epithelial layer was gently scraped free with a no. 15 scalpel blade. The cell suspension was transferred to a 50-mL tube and centrifuged at 1000×g for 4 minutes. Collected cells were transferred to a flask coated with a mixture of fibronectin and collagen (Sigma-Aldrich Corp.), containing 5 mL of serum-free CnT-20 medium.
Cell migration was assayed using the Transwell system (Corning Costar, Acton, MA, USA) with 6.5 mm-diameter polycarbonate filters (8-µm pore size). Briefly, the lower surface of the filter was coated with 10 µg/mL fibronectin (Sigma-Aldrich Corp.), 10 µg/mL recombinant human TGFBIp (rhTGFBIp) (Sino Biological Inc., Beijing, China), or bovine serum albumin at a concentration of 3% (w/v) as a control for nonspecific binding. SV40-CECs or primary CECs (105) were seeded onto chemotaxis filters in DMEM plus 1% fetal bovine serum. After the 5-hour migration period, non-migrating cells were removed and migrating cells were stained with hematoxylin and eosin (H&E) and quantified using Kodak 1D software (Eastman Kodak, Rochester, NY, USA). Results are representative of three different experiments in duplicate.
The wound-healing assay was performed by scratching confluent SV40-CECs or primary CECs on 35-mm dishes with micropipette tips. The cells were then washed to remove debris and treated with rhTGFBIp (10 µg/mL) for 16 hours. Images were captured at 0 and 16 hours after wounding. For quantitative analysis, five fields per plate were photographed, and distances between the front lines were measured using ImageJ software (National Institutes of Health). Each assay was repeated three times.
Cell-matrix adhesion assays were performed as described previously.36 The 96-well plates were coated with 10 µg/mL human fibronectin (Sigma-Aldrich Corp.) or 2.5–10 µg/mL rhTGFBIp. SV40-CECs or primary CECs in adhesion buffer (serum-free media: DMEM or CnT-20) were seeded at 105 cells/well in 100 µL volume and incubated for 30 minutes at 37℃. After two washes to remove non-adherent cells, adherent cells were stained with H&E and quantified in triplicate by counting adherent cells in five randomly selected fields per well (Axiovert 100; Carl Zeiss Micro-Imaging, Thornwood, NY, USA). Results are representative of three different experiments in duplicate.
CEC proliferation was assessed using MTT assays (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide) according to the manufacturer's protocol. Briefly, SV40-CECs or primary CECs were seeded onto 96-well plates and incubated with different concentrations of rhTGFBIp (2.5–10 µg/mL) for 72 hours. Fifteen microliters of MTT solution and 200 µL dimethyl sulfoxide were successively added to each well and the optical density measurement (490 nm) was performed. All groups of experiments were performed in quintuplicate.
SV40-CECs, primary CECs or conjunctival epithelial cells (2×105 cells/well) were seeded onto 60 mm plates and treated with TGFBIp (10 µg/mL) for 24 hours. Total RNA was isolated from cells by extraction in TRIZOL reagent (Invitrogen) at each time point (3, 6, 9, 12, and 24 hours). SV40-CECs (2×105 cells/well) were seeded onto 60 mm plates, After 24 hours, cells were pre-incubated for 40 minutes with or without PD98059 (10 µM) or Wortmannin (100 nM), then stimulated with TGFBIp (10 µg/mL) for 6 hours. Total RNA was isolated from cells by extraction in TRIZOL reagent (Invitrogen). Using the Power SYBR Green RNA-to-CT™ 1-Step kit (Applied Biosystems, Foster City, CA, USA) and StepOnePlus™ (Applied Biosystems), mRNA expression of the human glyceraldehyde 3-phosphate dehydrogenase (GAPDH), MUCIN1, MUCIN4, MUCIN5AC, MUCIN7, and MUCIN16 genes was measured according to the manufacturer's instructions. The qRT-PCR conditions for all genes were as follows: 48℃ for 30 minutes, 95℃ for 10 minutes, then 40 cycles of 95℃ for 15 seconds, and 60℃ for 1 minute. The results are based on cycle threshold (Ct) values. We calculated differences between the Ct values for experimental and reference genes (GAPDH) and graphed the results as the ratio of each RNA to the calibrator sample. Primers used for gene amplification were the following: MUCIN1 5′-AGCGTGAGTGATGTGCCATT-3′ (sense) and 5′-AGCGCA ACCAGAACACAGAC-3′ (antisense); MUCIN4 5′-GGTGGTG GAGGCGTTCTTAT-3′ (sense) and 5′-CTCACGTTCAGGGCT GTCAC-3′ (antisense); MUCIN5AC 5′-CGCTCAGCTGT TCTCTGGAC-3′ (sense) and 5′-GCACAGGTCGACTGGTTCT G-3′ (antisense); MUCIN7 5′-TGCCCCAATTACCACACCTA-3′ (sense) and 5′-TATTTTGGCCAGGAGCTGAA-3′ (antisense); MUCIN16 5′-CCAACTCTTCCGAAACAGCA-3′ (sense) and 5′-GCCAGTGGCGAGAAGTTACA-3′ (antisense); GAPDH 5′-A TGGGGAAGGTGAAGGTCG-3′ (sense), and 5′-GGGGTCATT GATGGCAACAATA-3′ (antisense). Three independent experiments were performed and statistical analysis was carried out using Newman-Keuls multiple comparison tests.
SV40-CECs (2×105 cells/well) were seeded onto 60 mm plates. After 24 hours, cells were pre-incubated for 40 minutes with or without PD98059 (10 µM) or Wortmannin (100 nM), and then stimulated with TGFBIp (10 µg/mL) for 8 hours. Growth medium was then removed, and the cells were rinsed twice with phosphate buffered saline prior to lysis with a radioimmunoprecipitation assay (RIPA) buffer. Cell lysates were electrophoresed on SDS-polyacrylamide gel electrophoresis (PAGE) and proteins were transferred onto polyvinylidene difluoride membranes. The blocked membranes were incubated with the appropriate antibody [anti-human MUCIN1, MUCIN4, or ACTIN (1:1000 dilution, Abcam Inc., Cambridge, MA, USA)], and the immunoreactive bands were visualized with a chemiluminescent reagent, as recommended by Amersham Biosciences, Inc. (Piscataway, NJ, USA).
SV40-CECs (2×105 cells/well) were seeded onto 60 mm plates. After 24 hours, cells were treated with TGFBIp (10 µg/mL) for the indicated period of time (5, 10, 15, 30, and 60 minutes). Cells were harvested with RIPA buffer and cell lysates were electrophoresed on SDS-PAGE and proteins were transferred onto polyvinylidene difluoride membranes. The blocked membranes were incubated with the appropriate antibody [anti-human pFAK, pSRC, pERK, pAKT, FAK, SRC, ERK, and AKT (1:1000 dilution, Cell Signaling Technology Inc., Danvers, MA, USA)], and the immunoreactive bands were visualized with an enhanced chemiluminescence immunoblotting system (GE Healthcare, Buckinghamshire, UK).
To assess the effect of TGFBIp on CEC activity, we first examined the effect of TGFBIp on cell migration of CECs. As shown in Fig. 1A and B, TGFBIp increased CEC migration in a dose-dependent manner. The magnitude of TGFBIp-induced CEC migration was higher than that induced by fibronectin, which is a known CEC migration-inducing factor (Fig. 1B).
Next, we analyzed the effect of TGFBIp on CEC adhesion and found that TGFBIp increased SV40-CEC adhesion in a dose-dependent manner (Fig. 1C and D). In addition, CECs stimulated with TGFBIp exhibited enhanced proliferation activity compared with the activity of unstimulated cells (Fig. 1E). This increased activity was replicated in the wound-healing assay, which showed that SV40-CECs migrated more into the scratch wound after TGFBIp treatment (Fig. 1F and G). We cultured primary CECs from human corneal epithelial tissue and performed migration, adhesion, proliferation, and wound healing assays. As shown in Fig. 2, TGFBIp increased primary CEC migration, adhesion, proliferation, and wound healing in a dose-dependent manner. Taken together, these results suggest that TGFBIp promotes migration, adhesion, and proliferation of CECs, which may enhance wound healing in these cells.
Next, we investigated whether TGFBIp promotes the expression of mucin genes in the corneal and conjunctival epithelial cells. As shown in Figs. 3A and 4A, TGFBIp treatment increased the expression of MUC1, -4, -5AC, and -16 in SV40-CECs and primary CECs, and maximal expression was observed after 6 hours, whereas the mRNA levels of MUC7 were unchanged. Furthermore, TGFBIp also increased the expression of MUC1, -4, -5AC, -7, and -16 genes in conjunctival epithelial cells (Supplementary Fig. 1A, only online). Basal expression levels of MUC16 among the mucin genes were highest in SV40 immortalized CECs, primary cultured human CECs, human corneal epithelial tissue, and conjunctival epithelial cells (Figs. 3B and 4B) (Supplementary Figs. 1B and 2, only online). These observations provide evidence that TGFBIp may have a protective effect on ocular surfaces by inducing the expression of mucin genes in corneal and conjunctival epithelial cells.
To determine which signaling pathways are involved in modulating expression of mucin genes in TGFBIp-stimulated SV40-CECs, we analyzed the activity of intracellular signaling molecules. As shown in Fig. 5A and B, phosphorylation of ERK and AKT signaling molecules was increased by TGFBIp stimulation in a time-dependent manner, whereas TGFBIp had no effect on FAK or SRC phosphorylation (Fig. 5A). To further investigate the significance of the ERK and AKT signaling pathway in regulating mucin expression in TGFBIp-stimulated CECs, CECs were pre-treated with PD98059, an ERK inhibitor, or Wortmannin, an AKT inhibitor, and then stimulated with TGFBIp. As shown in Fig. 5C, TGFBIp-stimulated CECs showed increased expression of MUC1, -4, and -16 mRNA, and this effect was effectively blocked by PD98059 or Wortmannin. Western blot analysis confirmed the inhibition of TGFBIp-induced ERK and AKT phosphorylation and of TGFBIp-induced MUC1 and -4 expression by PD98059 or Wortmannin (Fig. 5D, E, and F). These results suggest that ERK and AKT signaling are important in regulating the expression of mucin genes in TGFBIp-treated CECs.
In this study, we showed that TGFBIp promoted migration, adhesion, and proliferation of human CECs in a dose-dependent manner, and that these effects would mediate wound healing of CECs. These results suggest that TGFBIp secreted by CECs themselves may cause neighboring cells to activate wound-healing processes in an autocrine or paracrine manner.
Membrane-spanning and secretory mucins are critical to the health of the ocular surface and protection of this tissue from the external environment.67837 In this study, we demonstrated that TGFBIp increasesd the expression of MUC1, -4, -5AC, and -16 in CECs, whereas the expression of MUC7 was unchanged in response to TGFBIp. Furthermore, TGFBIp increased the expression of MUC1, -4, -5AC, -7, and -16 in conjunctival epithelial cells. Interestingly, basal expression of MUC16 was highest in SV40 immortalized CECs, CECs from human tissue, and conjunctival epithelial cells. This finding suggests that MUC16 is predominantly expressed in the ocular surface and may contribute to the health of the ocular surface.
TGFBIp also significantly increased the phosphorylatoin of ERK and AKT in CECs, and inhibition of ERK and AKT signaling via pretreatment with PD98059 or Wortmannin markedly abrogated TGFBIp-mediated mucin expression. The phosphorylation of FAK and Src remained unchanged with TGFBIp treatment. Previously, we reported that TGFBIp binds to integrin and activates the intracellular signaling molecules.3839 However, the subtypes of integrin that mediate interactions with TGFBIp vary depending on the cell types. TGFBIp binds to integrin αVβ3 in corneal fibroblasts and activates the endocytosis of TGFBIp itself,38 but in endothelial progenitor cells, TGFBIp activates the intracellular signaling molecules via binding to integrins a4 and a5 and induces differentiation of endothelial progenitor cells.39 Since TGFBIp-interacting proteins vary depending on the cell type and downstream signaling pathway, the final outcome may also differ in varying cell types. In our study, we did not investigate whether TGFBIp activated ERK and AKT through integrin binding in the cell types studied. In addition, FAK and Src activation is not always induced by integrin binding of ligands.40 Therefore, we suggest that TGFBIp may activate ERK and AKT with integrin binding and may not be able to induce the activation of FAK and Src independent of integrin binding in our cells.
Although TGFBIp is a secretory ECM protein, we found that the induction of mucins by TGFBIp is specifically ERK- and AKT-dependent. The relationship between TGFBIp signaling and mucin expression in CECs has been unclear until now. However, our present data indicate a direct link between TGFBIp signaling and mucin expression in CECs, therefore, being the first to describe an interrelation between TGFBIp and mucins.
In summary, our findings clearly showed that TGFBIp was a critical factor for the health of the ocular surface. First, TGFBIp increased migration, adhesion, proliferation, and wound healing of CECs. Second, TGFBIp activated the ERK and AKT signaling pathway, which in turn induced the expression of mucins in CECs. To the best of our knowledge, these data are the first to suggest that the locally generated TGFBIp in the cornea may contribute to wound healing of CECs by enhancing the migration, adhesion, and proliferation of CECs. In addition, TGFBIp may have a protective effect on the ocular surfaces by inducing the expression of mucin genes in corneal and conjunctival epithelial cells. Collectively, these results suggest that TGFBIp is a useful therapeutic target for patients with conditions such as corneal wounds.
Figures and Tables
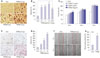 | Fig. 1TGFBIp increases the migration, adhesion, proliferation, and wound healing of SV40-CECs. (A and B) Effect of TGFBIp on SV40-CECs migration. SV40-CECs were treated with TGFBIp or fibronectin and migration was quantified. (C and D) Effect of TGFBIp on SV40-CECs adhesion. SV40-CECs were treated with TGFBIp and cell adhesion was quantified. (E) Effect of TGFBIp on SV40-CECs proliferation. SV40-CECs were incubated with TGFBIp (2.5–10 µg/mL) for 72 hours and cell proliferation was assessed via MTT assay. (F and G) Effect of TGFBIp on SV40-CECs wound healing. SV40-CECs were scratched with micropipette tips and treated with TGFBIp. Images were captured at 0 and 16 hours after wounding. All data are presented as mean±SE from three independent experiments performed in duplicates or quintuplicate. **p<0.01 vs. control or fibronectin. TGFBIp, transforming growth factor-β-induced protein; CECs, corneal epithelial cells; SE, standard error; Cont, control. |
 | Fig. 2TGFBIp increases the migration, adhesion, proliferation, wound healing of primary CECs. (A) Effect of TGFBIp on primary CECs migration. Primary CECs were treated with TGFBIp and migration was quantified. (B) Effect of TGFBIp on primary CECs adhesion. Primary CECs were treated with TGFBIp and cell adhesion was quantified. (C) Effect of TGFBIp on primary CECs proliferation. Primary CECs were incubated with TGFBIp (2.5–10 µg/mL) for 72 hours and cell proliferation was assessed via MTT assay. (D) Effect of TGFBIp on primary CECs wound healing. Primary CECs were scratched with micropipette tips and treated with TGFBIp. For quantitative analysis, distances between front lines were measured using the ImageJ software. All data are presented as mean±SE from three independent experiments performed in duplicates or quintuplicate. **p<0.01 vs. control. TGFBIp, transforming growth factor-β-induced protein; CECs, corneal epithelial cells; SE, standard error; Cont, control. |
 | Fig. 3TGFBIp increases the expression of mucins in SV40-CECs. (A) The temporal expression of each gene in SV40-CECs treated with TGFBIp was determined by qRT-PCR. (B) The mRNA level of each gene in SV40-CECs without TGFBIp treatment was determined by qRT-PCR. All results were normalized to GAPDH. **p<0.01 vs. control. TGFBIp, transforming growth factor-β-induced protein; CECs, corneal epithelial cells; qRT-PCR, quantitative reverse transcription PCR; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
 | Fig. 4TGFBIp treatment induces the expression of mucins in primary CECs. (A) The temporal expression of each gene in primary CECs treated with TGFBIp was determined by qRT-PCR. (B) The mRNA level of each gene in primary CECs without TGFBIp treatment was determined by qRT-PCR. All results were normalized to those for GAPDH. **p<0.01 vs. control. TGFBIp, transforming growth factor-β-induced protein; CECs, corneal epithelial cells; qRT-PCR, quantitative reverse transcription PCR; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
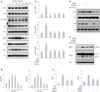 | Fig. 5TGFBIp regulates the expression of mucins through the ERK and AKT signaling pathways in CECs. (A) SV40-CECs were treated with TGFBIp (10 µg/mL) for indicated period of time and cell lysates were subjected to Western blot analysis. (B) The relative ratios were normalized by arbitrarily setting the phosphorylation ratio at time 0 as 1. Similar results were obtained from three additional experiments (**p<0.01 vs. time 0). (C) SV40-CECs were pre-incubated with or without PD98059 (10 µM) or Wortmannin (100 nM), then stimulated with TGFBIp (10 µg/mL) for 6 hours. The mRNA level of each gene in CECs was determined by qRT-PCR. All results were normalized to GAPDH. **p<0.01 vs. control or TGFBIp. (D) SV40-CECs were pre-incubated with or without PD98059 (10 µM) or Wortmannin (100 nM) and then stimulated with TGFBIp (10 µg/mL) for 10 minutes. Cell lysates were subjected to Western blot. (E and F) SV40-CECs were pre-incubated with or without PD98059 (10 µM) or Wortmannin (100 nM) and then stimulated with TGFBIp (10 µg/mL) for 8 hours. Protein levels of mucin 1 and mucin 4 were analyzed by Western blot analysis. The relative ratios were normalized by arbitrarily setting the expression ratio for the control as 1. Similar results were obtained from three additional experiments (**p<0.01 vs. TGFBIp). TGFBIp, transforming growth factor-β-induced protein; CECs, corneal epithelial cells; qRT-PCR, quantitative reverse transcription PCR; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
ACKNOWLEDGEMENTS
This research was supported by a basic science research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF 2014R1A1A2057458, NRF-2016R1D1A1B03933337), and by a faculty research grant of Yonsei University College of Medicine for 2007 (6-2007-0173).
References
1. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011; 52:1938–1978.

2. Gipson IK, Argüeso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003; 231:1–49.

3. Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004; 78:173–185.

4. Spurr-Michaud S, Argüeso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007; 84:939–950.

5. Carraway KL, Perez A, Idris N, Jepson S, Arango M, Komatsu M, et al. MUC4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol. 2002; 71:149–185.
6. Argüeso P. Glycobiology of the ocular surface: mucins and lectins. Jpn J Ophthalmol. 2013; 57:150–155.

8. Argüeso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009; 284:23037–23045.

9. Jonckheere N, Van Seuningen I. The membrane-bound mucins: from cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie. 2010; 92:1–11.

10. McGuckin MA, Quin RJ, Ward BG. Progesterone stimulates production and secretion of MUC1 epithelial mucin in steroid-responsive breast cancer cell lines. Int J Oncol. 1998; 12:939–945.

11. Treon SP, Mollick JA, Urashima M, Teoh G, Chauhan D, Ogata A, et al. MUC-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood. 1999; 93:1287–1298.

12. Theodoropoulos G, Carraway KL. Molecular signaling in the regulation of mucins. J Cell Biochem. 2007; 102:1103–1116.

13. Hori Y, Spurr-Michaud S, Russo CL, Argüeso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004; 45:114–122.

14. Ma M, Zhang Z, Niu W, Zheng W, Kelimu J, Ke B. Fibroblast growth factor 10 upregulates the expression of mucins in rat conjunctival epithelial cells. Mol Vis. 2011; 17:2789–2797.
15. Hori Y, Spurr-Michaud SJ, Russo CL, Argüeso P, Gipson IK. Effect of retinoic acid on gene expression in human conjunctival epithelium: secretory phospholipase A2 mediates retinoic acid induction of MUC16. Invest Ophthalmol Vis Sci. 2005; 46:4050–4061.

16. Seo KY, Chung SH, Lee JH, Park MY, Kim EK. Regulation of membrane-associated mucins in the human corneal epithelial cells by dexamethasone. Cornea. 2007; 26:709–714.

17. Li S, Bobek LA. Functional analysis of human MUC7 mucin gene 5′-flanking region in lung epithelial cells. Am J Respir Cell Mol Biol. 2006; 35:593–601.

18. Tei M, Moccia R, Gipson IK. Developmental expression of mucin genes ASGP (rMuc4) and rMuc5ac by the rat ocular surface epithelium. Invest Ophthalmol Vis Sci. 1999; 40:1944–1951.
19. Thapa N, Lee BH, Kim IS. TGFBIp/betaig-h3 protein: a versatile matrix molecule induced by TGF-beta. Int J Biochem Cell Biol. 2007; 39:2183–2194.
20. Gibson MA, Kumaratilake JS, Cleary EG. Immunohistochemical and ultrastructural localization of MP78/70 (betaig-h3) in extracellular matrix of developing and mature bovine tissues. J Histochem Cytochem. 1997; 45:1683–1696.

21. Escribano J, Hernando N, Ghosh S, Crabb J, Coca-Prados M. cDNA from human ocular ciliary epithelium homologous to beta ig-h3 is preferentially expressed as an extracellular protein in the corneal epithelium. J Cell Physiol. 1994; 160:511–521.

22. Billings PC, Herrick DJ, Kucich U, Engelsberg BN, Abrams WR, Macarak EJ, et al. Extracellular matrix and nuclear localization of beta ig-h3 in human bladder smooth muscle and fibroblast cells. J Cell Biochem. 2000; 79:261–273.

23. Karring H, Runager K, Valnickova Z, Thøgersen IB, Møller-Pedersen T, Klintworth GK, et al. Differential expression and processing of transforming growth factor beta induced protein (TGFBIp) in the normal human cornea during postnatal development and aging. Exp Eye Res. 2010; 90:57–62.

24. Kim JE, Kim EH, Han EH, Park RW, Park IH, Jun SH, et al. A TGF-beta-inducible cell adhesion molecule, betaig-h3, is downregulated in melorheostosis and involved in osteogenesis. J Cell Biochem. 2000; 77:169–178.

25. Bae JS, Lee SH, Kim JE, Choi JY, Park RW, Park JY, et al. Betaig-h3 supports keratinocyte adhesion, migration, and proliferation through alpha3beta1 integrin. Biochem Biophys Res Commun. 2002; 294:940–948.

26. Zhang Y, Wen G, Shao G, Wang C, Lin C, Fang H, et al. TGFBI deficiency predisposes mice to spontaneous tumor development. Cancer Res. 2009; 69:37–44.

27. Maeng YS, Aguilar B, Choi SI, Kim EK. Inhibition of TGFBIp expression reduces lymphangiogenesis and tumor metastasis. Oncogene. 2016; 35:196–205.

28. Park SW, Bae JS, Kim KS, Park SH, Lee BH, Choi JY, et al. Beta ig-h3 promotes renal proximal tubular epithelial cell adhesion, migration and proliferation through the interaction with alpha3beta1 integrin. Exp Mol Med. 2004; 36:211–219.

29. Kim YH, Kwon HJ, Kim DS. Matrix metalloproteinase 9 (MMP-9)-dependent processing of βig-h3 protein regulates cell migration, invasion, and adhesion. J Biol Chem. 2012; 287:38957–38969.

30. Fujiki K, Hotta Y, Nakayasu K, Yokoyama T, Takano T, Yamaguchi T, et al. A new L527R mutation of the betaIGH3 gene in patients with lattice corneal dystrophy with deep stromal opacities. Hum Genet. 1998; 103:286–289.

31. Streeten BW, Qi Y, Klintworth GK, Eagle RC Jr, Strauss JA, Bennett K. Immunolocalization of beta ig-h3 protein in 5q31-linked corneal dystrophies and normal corneas. Arch Ophthalmol. 1999; 117:67–75.

32. Runager K, Klintworth GK, Karring H, Enghild JJ. The insoluble TGFBIp fraction of the cornea is covalently linked via a disulfide bond to type XII collagen. Biochemistry. 2013; 52:2821–2827.

33. Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995; 36:614–621.
34. Lee H, Kim EK, Kim JY, Yang YM, Shin DM, Kang KK, et al. DA-6034-induced mucin secretion via Ca2+-dependent pathways through P2Y receptor stimulation. Invest Ophthalmol Vis Sci. 2014; 55:6565–6574.

35. Maldonado BA, Furcht LT. Epidermal growth factor stimulates integrin-mediated cell migration of cultured human corneal epithelial cells on fibronectin and arginine-glycine-aspartic acid peptide. Invest Ophthalmol Vis Sci. 1995; 36:2120–2126.
36. Maeng YS, Choi HJ, Kwon JY, Park YW, Choi KS, Min JK, et al. Endothelial progenitor cell homing: prominent role of the IGF2-IGF2R-PLCbeta2 axis. Blood. 2009; 113:233–243.

37. Situ H, Bobek LA. In vitro assessment of antifungal therapeutic potential of salivary histatin-5, two variants of histatin-5, and salivary mucin (MUC7) domain 1. Antimicrob Agents Chemother. 2000; 44:1485–1493.

38. Choi SI, Maeng YS, Kim TI, Lee Y, Kim YS, Kim EK. Lysosomal trafficking of TGFBIp via caveolae-mediated endocytosis. PLoS One. 2015; 10:e0119561.

39. Maeng YS, Choi YJ, Kim EK. TGFBIp regulates differentiation of EPC (CD133(+) C-kit(+) Lin(-) cells) to EC through activation of the Notch signaling pathway. Stem Cells. 2015; 33:2052–2062.

40. Huveneers S, Danen EH. Adhesion signaling-crosstalk between integrins, Src and Rho. J Cell Sci. 2009; 122(Pt 8):1059–1069.
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1
TGFBIp increases the expression of mucins in conjunctival epithelial cells. (A) The temporal expression of each gene in human conjunctival epithelial cells treated with TGFBIp was determined by qRT-PCR. (B) The mRNA level of each gene in human conjunctival epithelial cells without TGFBIp treatment was determined by qRT-PCR. All results were normalized to GAPDH. **p<0.01 vs. control. TGFBIp, transforming growth factor-β-induced protein; qRT-PCR, quantitative reverse transcription PCR; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download