Abstract
Purpose
The aim of this study was to investigate the anti-fibrotic effect of relaxin in subsynovial fibroblasts activated by transforming growth factor beta (TGF-β).
Materials and Methods
To test the anti-fibrotic effect of an adenovirus-relaxin construct (Ad-RLN) on subsynovial fibroblasts in vitro, cells from subsynovial connective tissue of patients with carpal tunnel syndrome were activated with TGF-β1 and exposed to Ad-RLN (as a therapeutic gene) or adenovirus-lacZ construct (as a marker gene) for four hours. Subsynovial fibroblast cultures without adenoviral exposure served as controls.
Results
We observed induction of gene expressions of collagen I, III and IV, as well as the abatement of alpha-smooth muscle actin (a-SMA) synthesis, Smad2 phosphorylation, and fibronectin at the protein level, in comparison to controls. In addition, protein expressions of matrix metalloproteinase (MMP) I was significantly induced, whereas the protein expressions of tissue inhibitor of metalloproteinases (TIMP) I and IV were reduced due to relaxin expression.
Carpal tunnel syndrome (CTS) is the most common peripheral nerve compression syndrome, and non-inflammatory fibrosis and thickening of the subsynovial connective tissue (SSCT) are common pathologic findings in CTS.1234 SSCT is an important and unique structure, similar to the paratenon, which surrounds the flexor tendons and median nerve in the carpal tunnel comprising all tissues between the visceral synovial sheath and the flexor tendons.25 SSCT is composed of multiple layers of collagen bundles that run parallel to the flexor tendons, which are interconnected by vertical fibers.6 Fibrosis of SSCT is pronounced next to the flexor tendons, suggesting that shear forces between the tendons and SSCT are associated with pathologic changes.78 Excessive and repetitive shear forces are thought to initiate a series of mechanical events, such as altered stiffness and SSCT permeability, increased volume of the carpal tunnel contents,69 elevated pressure,10 ischemia,11 and compression of the median nerve. In CTS patients, increased adherence or dissociation between the flexor tendons and SSCT may increase shear forces even further.12 These shear forces could induce fibrosis of SSCT, which hampers median nerve mobility in CTS patients.51013 Increased shear modulus and pull-out force are also reported at the tissue level in SSCT from CTS patients.1214
Fibrosis of SSCT is associated with an increase in transforming growth factor beta (TGF-β) activity, which can up-regulate both fibroblast proliferation and activation.215 TGF-β stimulates membrane-cytoskeletal structural protein formation, which mechanically generates and transmits contractile force to the extracellular matrix (ECM).215 TGF-β is also implicated in the synthesis of ECM through multiple signal transduction pathways.16 In addition, TGF-β exerts ECM-preserving actions by suppressing the activity of matrix metalloproteinases (MMPs) and also by inducing synthesis of protease inhibitors, such as plasminogen activator inhibitor-1 and tissue inhibitor of metalloproteinase (TIMP).17
Relaxin (RLN), a member of the insulin superfamily, is a substance that influences the reproductive tract.18 RLN inhibits fibrosis and inflammatory activities at multiple levels, through activation of G-protein-coupled receptor, and also inhibits inflammatory cell influx and ameliorates the effects of profibrotic factors, such as TGF-β, injured organs.19
The anti-fibrogenic effect of RLN20 might provide a therapeutic target for the treatment of CTS, via the interwoven pathways of MMPs, TIMPs, collagenesis, and collagenolysis. With proven efficacy at the molecular level, RLN therapy might prevent the progression of SSCT fibrosis, or even resolve fibrosis, using a minimally invasive injection, depending on the stage of CTS. Therefore, this study aimed to investigate the anti-fibrotic effect of RLN, activated by TGF-β1, on subsynovial fibroblast of CTS patients.
The Human Subjects Institutional Review Board approved all of the experimental protocols in this study.
To test the anti-fibrotic effect of the adenovirus-relaxin construct (Ad-RLN) on synovial fibroblasts in vitro, we utilized cells from the tissues of patients with CTS. Synovial fibroblasts were activated by TGF-β1 (2 ng/mL), exposed to Ad-RLN as a therapeutic gene and adenovirus-lacZ construct (Ad-LacZ) as a marker gene, and cultured for four hours. Synovial fibroblast cultures, without adenoviral exposure, served as the control. The mRNA expression levels of collagen types I, III, IV, V, and XI, as well as MMP-1, -3, -8, -9, and -13 were analyzed by reverse-transcription polymerase chain reaction. In addition, protein expression levels of fibronectin, phosphorylation of Smad2 and ERK1/2, and alpha smooth muscle actin (α-SMA) were estimated using western blot analysis. Finally, total collagen synthesis was assayed.
The SSCT were harvested from seven patients with CTS during open carpal tunnel release. Minced tissues were digested at 37℃ under gentle agitation with type IV collagenase (250 unit/mL; Sigma, St. Louis, MO, USA). Cells were placed in culture plates (TPP®, Trasadingen, Switzerland) at 4×105 cells/mL. Primary cultures were sustained for two to three weeks in Dulbecco's Modified Eagle Medium (Life Technologies Corporation) containing 10% fetal bovine serum, 1% v/v penicillin, streptomycin, and nystatin (Life Technologies Corporation), in a 37℃ incubator with 5% CO2 and humidity.
For this study, Ad-LacZ expressing the lacZ gene was used as a viral control, and Ad-RLN expressing the human RLN gene was also prepared. Each recombinant adenoviral vector originated from replication-deficient adenovirus type 5 lacking E1 and E3 regions. The RLN gene was cloned into the E1 region under the control of the human cytomegalovirus early promoter.21 Recombinant virus was grown in transformed human embryonic kidney 293 cells and purified with VIVAPURE ADENOPACK 100 (Sartorius Stedim biotech, Goettingen, Germany). Titers were determined by optical density at 260 nm.21 At confluence, the subsynovial fibroblastic cells were rinsed with Dulbecco's Phosphate Buffered Saline (Life Technologies Corporation), and then exposed to Dulbecco's Modified Eagle Medium (DMEM) containing one dose of Ad-LacZ or Ad-RLN with a viral concentration of 80 multiplicity of infection.
Total RNA was isolated from the subsynovial fibroblasts transfected with Ad-RLN, using the QIAGEN RNeasy® mini kit (QIAGEN, Germantown, MD, USA). In addition, cDNA was prepared using the QuantiTect® Reverse Transcription Kit (QIAGEN, CA, USA). Collagen and MMP genes were amplified, while relative expressions were normalized to the average beta-actin levels. Resulting data were analyzed using the ImageJ software, Ver. 1.6 (National Institutes of Health, Bethesda, MD, USA).
Cells (2×105 per well) were transfected with Ad-RLN for four hours, and lysed in RIPA lysis buffer (ATTO Corp., Tokyo, Japan) containing protease inhibitor (Pierce, Rockford, IL, USA). Meanwhile, the culture medium was analysed for RLN protein expression by Ad-RLN. Lysates and culture medium were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were then transferred to polyvinylidenedifluoride (Pierce) membranes using a transfer system (Mini Trans-Blot® Cell and systems, Bio-Rad, Hercules, CA, USA). The blots were incubated with specific antibodies against MMP-1, MMP-13 (Young In Frontier Co., Ltd., Seoul, Korea), ERK1/2, phosphor-ERK1/2 Smad2, phosphor-Smad2 (Cell Signaling Technology, Danvers, MA, USA), beta galactosidase, α-SMA, fibronectin, TIMP 1, and TIMP 4 (Abcam®, Cambridge, UK). After reacting with secondary antibodies, immunoreactive bands were visualized by a western blot detection system (ATTO Corp.). To verify the amounts of loaded proteins, blots were stripped of bound antibodies and re-probed using antibodies to actin (Abcam®).
In the DMEM medium, cells transfected with Ad-RLN were grown at a density of 4×104 cells per well on 35-mm culture plates for four hours. Collagen was harvested from these cells, after lysing them in a buffer containing 0.5 M acetic acid and protease inhibitors. The collagen samples were concentrated according to an isolation and concentration protocol, and the amount was then measured spectrophotometrically at 555 nm (Biocolor LTD., Carrickfergus, UK).
All data are expressed as mean±standard deviation (SD), compiled from three independent experiments of separate cultures isolated from seven donors. Two-tailed Student's t-test was used to compare the means of the two groups. In our analyses, a value of p<0.05 was considered statistically significant.
Western blot analysis of beta-galactosidase and RLN protein expressions in subsynovial fibroblasts from CTS patients transfected with either Ad-LacZ or Ad-RLN indicated a highly efficient transduction rate for the adenoviral marker gene construct (Fig. 1).
The mRNA expressions of collagen types I and III in the subsynovial fibroblasts of CTS patients transfected with Ad-RLN decreased by 60% and 20%, respectively, compared to the control culture with TGF-β1 and Ad-LacZ (*:p<0.05, †: p<0.001), whereas that of collagen type IV increased 30%, compared to the control culture with TGF-β1 and Ad-LacZ (p<0.05) (Fig. 2). On the other hand, however, no significant difference was found in the expressions of collagen type V, at mRNA level (*: p<0.05, †: p<0.001).
Furthermore, at the four-hour mark, subsynovial fibroblasts from CTS patients transfected with Ad-RLN showed increases of 30, 10, 80, and 40% in MMP-1, -3, -8, and -9 mRNA expressions, respectively, compared to the control culture with TGF-β1 and Ad-LacZ (*: p<0.05, †: p<0.001) (Fig. 3).
Subsynovial fibroblasts from CTS patients with Ad-RLN showed a 60% increase in the level of phospho-ERK1/2 protein expression after four hours, compared to cells cultured with TGF-β1 and Ad-LacZ (p<0.05). Meanwhile, phospho-Smad2 protein expression showed a 60% decrease under identical condition to the above (p<0.001) (Fig. 4).
Subsynovial fibroblasts from CTS patients with Ad-RLN showed a 45% decrease in TIMP 1 expression and a 55% decrease in TIMP 4 expression after four hours, compared to the control culture with TGF-β1 and Ad-LacZ (p<0.001) (Fig. 5).
Subsynovial fibroblasts from CTS patients transfected with Ad-RLN showed a 60% decrease in the level of α-SMA expression at four hours, compared to cells cultured with TGF-β1 and transfected with Ad-LacZ (p<0.05). Fibronectin protein expression in the Ad-RLN transfected cells decreased by 40%, compared to cells cultured with TGF-β1 and Ad-LacZ (p<0.001) (Fig. 6).
Subsynovial fibroblasts from CTS patients transfected with Ad-RLN showed a 15% increase in total collagen protein expression at four hours, compared to the control culture with TGF-β1 and Ad-LacZ (*: p<0.05, †: p<0.001) (Fig. 7).
The SSCT, a net-like connective tissue, connects the finger flexor tendons and median nerve and acts as a sliding unit to decrease friction during finger movement.422 A non-inflammatory fibrotic change and thickening of the SSCT are known to initiate an elevation of pressure in the carpal tunnel, causing neuropathy in idiopathic CTS.2422 In recent studies, synovial edema and synovial vascular proliferation were observed in patients with CTS.23 Myofibroblast and collagen type IV expression were detected in patients with idiopathic CTS, both of which are involved in the fibrotic process without relevance of inflammation.24 The exact mechanisms of SSCT fibrosis are unknown, although fibrosis in SSCT is a common histologic finding in idiopathic CTS.224 Furthermore, general consensussuggest that collagenous structure is changed by continuous exposure to shear stress,25 which induces overexpression in collagens and ECM components, and results in overall volume reduction and compression of the median nerve.23 Also, increase of collagen type IV in SSCT refers to accretion in the basal lamina, which leads to vascular proliferation and fibrosis.26 In addition, increased stiffness in human SSCT may cause fibroblast to transform into myofibroblast. In CTS animal model, overexpression of TGF-β1 and connective tissue growth factor (CTGF) were identified in the SSCT with shear injury.27
Normally, both expression and suppression of TGF-β1 and CTGF are found in the course of wound healing.28 However, repetitive injury promotes fibrosis in tissue and ECM, as well as profibrotic growth factor such as TGF-β1.29 TGF-β1 stimulates fibroblast, accumulates ECM, and downregulates MMPs and collagenases,30 resulting in a vicious circle of TGF-β1 that initiates fibrotic signaling, including increased matrix deposition.27 Many previous studies showed that the expressions of TGF-β and its receptors increased with SSCT fibrosis in CTS.22731 In addition, SSCT fibrosis is marked by the following events: increased activation of canonical TGF-β second messenger Smads,3132 increased expression of downstream fibrotic mediators such as CTGF,2731 increased production of collagen types I, III, and IV,23133 and decreased expression of matrix metalloproteases.153133
When conservative treatment fails to relieve the patient's symptoms of CTS, surgical decompression of the median nerve is usually indicated.34 Although release of the median nerve is a simple and effective procedure, complications such as wound infection, scar and pillar area pain, injuries to the nerve and flexor tendons, and rarely incomplete release have been reported. If an alternative effective non-operative local treatment is available, it should be considered for this compressive neuropathy.
RLN acts also as an antagonist for fibrosis, and promotes wound-healing, neo-angiogenesis, and vasodilation.193536 It also affects both contraction and relaxation of ligaments around the pelvis for the maintenance of pregnancy and parturition.1837 RLN has been shown to promote matrix remodeling by increasing cell proliferation, reducing α-SMA expression, and decreasing collagen synthesis in renal fibroblasts.38 Furthermore, recombinant human RLN can alter the connective tissue phenotype of human lung fibroblast, decrease overexpression of procollagen type I and III induced by TGF-β1, and reduce both synthesis and secretion of MMP-1.39 When exposed to RLN, human dermal fibroblasts can modulate secretion of MMPs and collagenase inhibitors.40 It has earlier been suggested that the RLN gene renders antifibrogenic effects on myofibroblastic cells from Dupuytren's nodule via a direct inhibition of collagen synthesis, not through a collagenolytic pathway, such as MMP-1 or -13 and TIMP 1 or 3.20
In the present study, we transformed subsynovial fibroblast to myofibroblast with TGF-β1, and investigated the effects of RLN on anti-fibrosis using genetic modification of subsynovial fibroblastic cells from patients with CTS using Ad-RLN. The mRNA expression levels of MMPs significantly increased after RLN transgene expression. In particular, the expression levels of MMP-1, -3, -8, and -9 increased, whereas the protein expression levels of TIMP 1 and TIMP 4 decreased by approximately 45% and 55%, respectively, compared to the control culture with TGF-β1 and Ad-LacZ. In particular, the synthesis of collagen type IV, a marker associated with vascular wall thickness, was reduced by RLN, implying that RLN can inhibit vascular proliferation. These observations suggest that RLN can act on the process of collagenolysis, in addition to the synthesisof collagen, and that RLN gene can influence both collagenesis as well as collagenolysis in CTS patients. In addition, RLN decreased α-SMA expression by 60% and fibronectin expression by 45%, both of which are the main components of ECM. Also, RLN decreased phosphorylation of Smad by approximately 60%. Therefore, a possibility exists that RLN can block the TGF-β1-Smad-ERK pathway.
There are a few limitations on this study: 1) we did not assess whether RLN affected subsynovial fibroblast without TGF-β1. 2) We did not confirm basal TGF-β1 expression in the tissue. Lastly, 3) we did not evaluate whether RLN acted on the individual fibroblast from CTS and dupuytren disease through the same mechanism involved in anti-fibrosis.
In conclusion, RLN prevents excessive synthesis of ECM by reducing expressions of its components, such as fibronectin, α-SMA, and phosphorylated Smad2, by increasing expression of MMPs; and by decreasing expression of TIMPs. Nevertheless, additional studies on subsynovial fibroblasts are necessary, since RLN can act in a complicated manner. Moreover, in-depth investigation on RLN behavior in ECM remodeling should be continued.
Figures and Tables
 | Fig. 1Subsynovial fibroblasts from patients with carpal tunnel syndrome were transfected with Ad-RLN and Ad-LacZ. The expressions of beta-galactosidase and relaxin compared to cells transfected with control with TGF-β1 or treated with SB505124, a TGF-β inhibitor, indicate a highly efficient transduction rate for the adenoviral construct. TGF-β1, transforming growth factor beta 1; Ad-RLN, adenovirus-relaxin gene construct; Ad-LacZ, adenovirus LacZ gene construct. |
 | Fig. 2Collagen mRNA expression in fibroblasts from patients with carpal tunnel syndrome transfected with Ad-RLNs. Collagen type I, III, and IV expressions were decreased in the cells transfected with Ad-RLN compared to control with TGF-β1 and Ad-LacZ. *p<0.05, †p<0.001. TGF-β1, transforming growth factor beta 1; Ad-RLN, adenovirus-relaxin gene construct; Ad-LacZ, adenovirus LacZ gene construct. |
 | Fig. 3mRNA expressions of matrix metalloproteinases in subsynovial fibroblasts from patients with carpal tunnel syndrome transfected with Ad-RLN. MMP-1, -3, -8, -9, and -13 expressions increased in the cells transfected with Ad-RLN compared to control with TGF-β1 and Ad-LacZ. *p<0.05, †p<0.001. TGF-β1, transforming growth factor beta 1; Ad-RLN, adenovirus-relaxin gene construct; Ad-LacZ, adenovirus LacZ gene construct. |
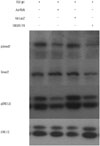 | Fig. 4Phospho-ERK and phospho-Smad2 protein expressions in subsynovial fibroblasts from patients with carpal tunnel syndrome when transfected with Ad-RLN. ERK1/2 and Smad2 phosphorylation decreased in the cells transfected with Ad-RLN compared to control with TGF-β1 and Ad-LacZ. p<0.001. TGF-β1, transforming growth factor beta 1; Ad-RLN, adenovirus-relaxin gene construct; Ad-LacZ, adenovirus LacZ gene construct. |
 | Fig. 5TIMPs and MMPs protein expression in subsynovial fibroblasts from patients with carpal tunnel syndrome when transfected with Ad-RLN. The expressions of TIMP 1 and TIMP 4 decreased whereas MMP-1 expression increased in the cells transfected with Ad-RLN compared to control with TGF-β1 and Ad-LacZ. p<0.001. TGF-β1, transforming growth factor beta 1; TIMP, tissue inhibitor of metalloproteinase; MMP, matrix metalloproteinase; Ad-RLN, adenovirus-relaxin gene construct; Ad-LacZ, adenovirus LacZ gene construct. |
 | Fig. 6Alpha smooth muscle actin and fibronectin protein expression in subsynovial fibroblasts from patients with carpal tunnel syndrome when transfected with Ad-LacZ. Alpha smooth muscle actin and fibronectin decreased in the cells transfected with Ad-RLN compared to control with TGF-β1 and Ad-LacZ. p<0.001. TGF-β1, transforming growth factor beta 1; RLN, relaxin; α-SMA, alpha smooth muscle actin; Ad-RLN, adenovirus-relaxin gene construct; Ad-LacZ, adenovirus LacZ gene construct. |
 | Fig. 7Total collagen expression in subsynovial fibroblasts from patients with carpal tunnel syndrome when transfected with Ad-RLN. Collagen expression decreased in the cells transfected with Ad-RLN compared to control with TGF-β1 and Ad-LacZ. *p<0.05, †p<0.001. TGF-β1, transforming growth factor beta 1; Ad-RLN, adenovirus-relaxin gene construct; Ad-LacZ, adenovirus LacZ gene construct. |
ACKNOWLEDGEMENTS
We would like to thank Chae-Ok Yun, Ph.D. (Department of Bioengineering, College of Engineering, Hanyang University, Seoul, Korea) for providing the adenovirus-relaxin construct. This work was supported, in part, by the Brain Korea 21 PLUS Project for Medical Science at Yonsei University.
This study was supported by a faculty research program from Yonsei University College of Medicine (6-2014-0098), as well as the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (grant number: 2011-0014399).
References
1. Armstrong TJ, Castelli WA, Evans FG, Diaz-Perez R. Some histological changes in carpal tunnel contents and their biomechanical implications. J Occup Med. 1984; 26:197–201.
2. Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004; 86-A:1458–1466.

3. Nakamichi K, Tachibana S. Histology of the transverse carpal ligament and flexor tenosynovium in idiopathic carpal tunnel syndrome. J Hand Surg Am. 1998; 23:1015–1024.

4. Neal NC, McManners J, Stirling GA. Pathology of the flexor tendon sheath in the spontaneous carpal tunnel syndrome. J Hand Surg Br. 1987; 12:229–232.

5. Oh J, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Immunolocalization of collagen types in the subsynovial connective tissue within the carpal tunnel in humans. J Orthop Res. 2005; 23:1226–1231.

6. Ettema AM, Amadio PC, Zhao C, Wold LE, O'Byrne MM, Moran SL, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006; 118:1413–1422.

7. Schuind F, Ventura M, Pasteels JL. Idiopathic carpal tunnel syndrome: histologic study of flexor tendon synovium. J Hand Surg Am. 1990; 15:497–503.

8. Ettema AM, Zhao C, An KN, Amadio PC. Comparative anatomy of the subsynovial connective tissue in the carpal tunnel of the rat, rabbit, dog, baboon, and human. Hand (N Y). 2006; 1:78–84.

9. Jinrok O, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Vascular pathologic changes in the flexor tenosynovium (subsynovial connective tissue) in idiopathic carpal tunnel syndrome. J Orthop Res. 2004; 22:1310–1315.

10. Zhao C, Ettema AM, Berglund LJ, An KN, Amadio PC. Gliding resistance of flexor tendon associated with carpal tunnel pressure: a biomechanical cadaver study. J Orthop Res. 2011; 29:58–61.

11. Keir PJ, Rempel DM. Pathomechanics of peripheral nerve loading. Evidence in carpal tunnel syndrome. J Hand Ther. 2005; 18:259–269.
12. Ettema AM, An KN, Zhao C, O'Byrne MM, Amadio PC. Flexor tendon and synovial gliding during simultaneous and single digit flexion in idiopathic carpal tunnel syndrome. J Biomech. 2008; 41:292–298.

13. Yamaguchi T, Osamura N, Zhao C, Zobitz ME, An KN, Amadio PC. The mechanical properties of the rabbit carpal tunnel subsynovial connective tissue. J Biomech. 2008; 41:3519–3522.

14. Osamura N, Zhao C, Zobitz ME, An KN, Amadio PC. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin Biomech (Bristol, Avon). 2007; 22:999–1003.

15. Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002; 118:211–215.

16. Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007; 35(Pt 4):661–664.
17. Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005; 117:69–80.
18. Schwabe C, Büllesbach EE. Relaxin, the relaxin-like factor and their receptors. Adv Exp Med Biol. 2007; 612:14–25.

19. Formigli L, Francini F, Nistri S, Margheri M, Luciani G, Naro F, et al. Skeletal myoblasts overexpressing relaxin improve differentiation and communication of primary murine cardiomyocyte cell cultures. J Mol Cell Cardiol. 2009; 47:335–345.

20. Kang YM, Choi YR, Yun CO, Park JO, Suk KS, Kim HS, et al. Down-regulation of collagen synthesis and matrix metalloproteinase expression in myofibroblasts from Dupuytren nodule using adenovirus-mediated relaxin gene therapy. J Orthop Res. 2014; 32:515–523.

21. Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006; 98:1482–1493.

22. Yoshii Y, Zhao C, Schmelzer JD, Low PA, An KN, Amadio PC. The effects of hypertonic dextrose injection on connective tissue and nerve conduction through the rabbit carpal tunnel. Arch Phys Med Rehabil. 2009; 90:333–339.

23. Tekin F, Sürmeli M, S¸ims¸ek H, Ceran C, Tezcan S, Taner ÖF, et al. Comparison of the histopathological findings of patients with diabetic and idiopathic carpal tunnel syndrome. Int Orthop. 2015; 39:2395–2401.

24. Yeşil M, Bacakaoğlu AK, Dogğan M. Are myofibroblasts activated in idiopathic carpal tunnel syndrome? an immunohistochemical study. Eklem Hastalik Cerrahisi. 2014; 25:133–140.

25. Tat J, Wilson KE, Keir PJ. Pathological changes in the subsynovial connective tissue increase with self-reported carpal tunnel syndrome symptoms. Clin Biomech (Bristol, Avon). 2015; 30:360–365.

26. Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009; 11:120–126.

27. Chikenji T, Gingery A, Zhao C, Passe SM, Ozasa Y, Larson D, et al. Transforming growth factor-β (TGF-β) expression is increased in the subsynovial connective tissues of patients with idiopathic carpal tunnel syndrome. J Orthop Res. 2014; 32:116–122.

28. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008; 16:585–601.
29. Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994; 331:1286–1292.
30. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004; 18:816–827.
31. Gingery A, Yang TH, Passe SM, An KN, Zhao C, Amadio PC. TGF-β signaling regulates fibrotic expression and activity in carpal tunnel syndrome. J Orthop Res. 2014; 32:1444–1450.

32. Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991; 111:131–143.

33. Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001; 276:17058–17062.

34. Ucan H, Yagci I, Yilmaz L, Yagmurlu F, Keskin D, Bodur H. Comparison of splinting, splinting plus local steroid injection and open carpal tunnel release outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int. 2006; 27:45–51.

35. Samuel CS, Lin F, Hossain MA, Zhao C, Ferraro T, Bathgate RA, et al. Improved chemical synthesis and demonstration of the relaxin receptor binding affinity and biological activity of mouse relaxin. Biochemistry. 2007; 46:5374–5381.

36. van der Westhuizen ET, Halls ML, Samuel CS, Bathgate RA, Unemori EN, Sutton SW, et al. Relaxin family peptide receptors--from orphans to therapeutic targets. Drug Discov Today. 2008; 13:640–651.
37. Hisaw FL. Experimental relaxation of the pubic ligament of the guinea pig. Proc Soc Exp Biol Med. 1926; 23:661–663.

38. Masterson R, Hewitson TD, Kelynack K, Martic M, Parry L, Bathgate R, et al. Relaxin down-regulates renal fibroblast function and promotes matrix remodelling in vitro. Nephrol Dial Transplant. 2004; 19:544–552.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download