Abstract
Purpose
To determine the influence of different ratios of hydroxyapatite (HA)/beta tricalcium phosphate (β-TCP) and collagen augmentation for posterior lumbar fusion in a rat model.
Materials and Methods
We generated a posterior lumbar fusion model in 50 rats and divided it into five groups of equal number as follows; 1) autologous bone graft as group A, 2) 70% HA+30% β-TCP as group B, 3) 70% HA+30% β-TCP+collagen as group C, 4) 30% HA+70% β-TCP as group D, and 5) 30% HA+70% β-TCP+collagen as group E. Rats were euthanized at 12 weeks after surgery and fusion was assessed by manual palpation, quantitative analysis using microCT and histology.
Results
The score of manual palpation was significantly higher in group C than group E (3.1±1.1 vs. 1.8±0.8, p=0.033). However, in terms of microCT analysis, group D showed significantly higher scores than group B (5.5±0.8 vs. 3.1±1.1, p=0.021). According to quantitative volumetric analysis, 30% HA+70% β-TCP groups (group D and E) showed significantly reduced fusion mass at 12 weeks after surgery (123±14.2, 117±46.3 vs. 151±27.3, p=0.008, 0.003, respectively). Collagen augmentation groups revealed superior results in terms of both microCT score and histologic grade.
Spinal bone fusion based surgery is a fundamental treatment for degenerative or deformed spinal disorder,1234 and autogenous bone grafting is considered golden standard as it shows osteoinductive, osteoconductive, and osteogenic properties.5 Recently, however, because of various complications after autogenous bone harvest, such as donor site pain, infection, and hematoma, another options for graft material have been introduced.678910 Also, we could not use autograft due to poor bone quality of elderly patients, osteoporosis, malignancy, and previously extracted donor site for primary fusion based operation.1112 Thus, for several decades, therefore, we have explored materials to substitute autograft such as growth factors, allograft, demineralized bone matrix, bone morphogenic proteins (BMPs), and stem cell transplantation.1314151617
Biphasic calcium phosphates (BCPs) were introduced in the late 1980s.18 They are composed of hydroxyapatite (HA), the most stable calcium orthophosphate and beta tricalcium phosphate (β-TCP), a more soluble compound, with varying HA/β-TCP ratios.192021 Bioactivity and resorption of BCPs can be different depending on the HA/β-TCP ratio and the crystallinity of the ceramic.222324 The effect of various HA/β-TCP ratios on bone fusion have been studied, especially in calvarial bone defect, and maxillary bony sinus.222324 Lim, et al.25 reported that the volume of new bone formation was not different between 7:3 HA/β-TCP group and 3:7 HA/β-TCP group and thus concluded that 3:7 HA/β-TCP ratio BCPs can be successfully used for sinus augmentation,25 and Ebrahimi, et al.26 also concluded that higher β-TCP ratios had beneficial effects during the early phase of cell proliferation and differentiation, whereas high HA ratio performed better in the later stage.
However, to the best of our knowledge, there were few studies on the effect of different ratio of BCPs on spinal fusion with microCT and histologic analysis although there are numerous articles about the efficacy for filling bony defect of calvarial, long bone, and maxillary sinus.12342627282930313233 Spinal fusion is not same as calvarial or maxillary sinus bone fusion due to its segmental mobility during the fusion process, and demands enough strength to support body weight. Recent trends reflect a desire to apply a lower HA and higher β-TCP proportions for more new bone formation, however, in cases of filling a bone defect, excessive absorption of β-TCP could have a worse effect on bone fusion, especially between mobile separate segments like consecutive vertebrae. This animal study using a rat fusion model was designed to investigate the effect of two different HA/β-TCP ratios (7:3 vs. 3:7) and the influence of collagen augmentation on posterior spinal fusion.
All procedures were conducted in accordance with the National Institute of Health's Guide for the Care and Use of Animals and were approved by the local Ethical Committee. For a posterior spinal fusion model of lumbar vertebrae, 50 male Sprague Dawley rats were divided into five groups by implanting the following materials between transverse processes of vertebrae, 1) autologous bone extracted rat tail bone as group A, 2) 70% HA+30% β-TCP as group B, 3) 70% HA+30% β-TCP+ collagen as group C, 4) 30% HA+70% β-TCP as group D, and 5) 30% HA+70% β-TCP+collagen as group E. Detailed data of each group were descripted in Table 1.
All 7:3 HA: β-TCP materials used in group B and 3:7 HA: β-TCP materials used in group D were HA coated with β-TCP with 0.3−0.5 mm sized particles and 300−500 µm porosity. 7:3 HA:β-TCP used in group B was granule type with 0.5 cc (approx. 500 mm3), and 7:3 HA: β-TCP+collagen, 3:7 HA: β-TCP, and 3:7 HA: β-TCP+collagen used in group C, D, and E were manufactured by the company which has same sized cylindrical shape as 6 mm diameter with 10 mm height (Ø 6×10 mm3×2 pieces=565 mm3), 0.3−0.5 mm sized particles, and 250 µm sized porosity. Augmented collagen was composed of type I collagen originated from bovine tendon.
Animals were anesthetized with intraperitoneal ketamine injection (80−100 mg/kg). A single dose of antibiotic (gentamicin at the dose of 0.05 mL/kg) was injected immediately after surgery prophylactically. Then, the animals were placed prone to a small operating table with warm heated and shaved, and the operation side was sterilized with a 10% povidone iodine solution. To harvest a bone graft from the tail, the tail was amputated and all soft tissue was removed from the bone using forceps, rongeurs and scalpels. Four vertebrae were separated from the intervertebral discs and all periosteum was removed. The bone was morselized with rongeurs and weighed to create a homogenous distribution of the grafts between the groups. Approximate volume of autologous tail bone used in group A was 474.9 mm3 (Ø mean 5.5×5 mm height×4 pieces).
After harvesting the tail bone graft, shaving, and disinfecting, a dorsal midline skin incision and a median fascial incision were made by scalpel and the paraspinal muscles were retracted to expose spinous process, lamina, and facet joint. We did not use electric drill, but used scalpel for decortication. The implant was cylindrical shape manufactured by company, thus the volumes of all the implants were exactly same. For group A, autogenously morselized bone harvested from the tail described above was placed on the posterior decorticated lamina surface at both sides. For BCP groups (groups B–E), two cylindrical materials were implanted on the posterior decorticated lamina surface at both side. The wounds were then closed with 3-0 nylon sutures. The rats recovered from anesthesia in the warm basket for 15 minutes and were returned to home cages. For postoperative pain, lidocaine 0.1 mg/kg was given every 6 to 12 hours for the first 2 postoperative days. Then, animals were observed twice daily throughout the post-surgical study period for general attitude, appetite, appearance of the surgical site, neurological signs and respiratory stress. Twelve weeks after surgery, the rats were euthanized by overdose of ketamine. After euthanasia, the lumbar spines were explanted and the soft tissues removed. Fusion was assessed by manual palpation, microCT evaluation [SkyScan1173 (SKYSCAN, Kontich, Belgium)] and histology.
Harvested lumbar spines were manually palpated by flexion and extension at the fusion level, and solidity was compared with the adjacent level by four different observers. Any motion detected on either side between the segments was considered ‘nonunion’ and it's score was 0. The absence of motion in every direction was determined as ‘union’ and it's score was 1. Four observers judged the scores according to the above rule. The four reviewers' scores were summed (maximum score, 4) and used them as variable for statistical analysis.
MicroCT scanning and analyses were performed on SkyScan1173 (SKYSCAN, Kontich, Belgium) using the manufacturer's analysis software. Serial axial microCT images from each specimen were graded, in a masked fashion, by three independent scorers according to the following scoring criteria: 0 if minimal or no evidence of bone formation was observed; 1 if immature bone formation with questionable fusion was observed and 2 if solid appearing bone with fusion likely.34 The 3 reviewers' scores were summed (maximum score, 6). A score of 5 or 6 was considered as indicating fusion.34
The scan was performed in the long axis of the spine with energy of 130 kV and a current of 30 µA, and a 250-ms exposure time producing 1200 axial images with 27.70 µm pixel sizes. To measure the total bone (new bone and residual graft material) and new bone volumes at the region of posterior spinal fusion, the axial image was converted to 3D by Digital Imaging and Communications in Medicine software (Lucion; Infinite, Seoul, Korea). We created three-dimensional image reconstructions to measure the total volume of fusion mass in both sides for each specimen.
After microCT analysis and manual palpation test, explanted spines were decalcified by 10% decalcifying solution HCL (Cal-Ex) (Fischer Scientific, Fairlawn, NJ, USA) and were fixed in 75% ethanol solution. Three blinded independent observers graded goldner's trichrome stained sections on a scale from 1 to 10 based on the histologic ratio of fibrous tissue, cartilage, and mature bone visualized on a low-power field, followed by the previously established method (Table 2).35
Statistical analysis was performed using SPSS version18.0.0 software (SPSS Inc., Chicago, IL, USA). Values were recorded as median (Q1, Q3). Differences in manual palpation grade, microCT score, histologic grade, and the volume of fusion mass among five groups were compared with the Kruskal-Wallis test. Post hoc analysis was performed by the Dunn procedure and Mann-Whitney U test. p<0.05 was considered statistically significant.
Data are provided in Table 3. With regard to manual palpation, group E showed significantly lower score [2 (1, 2)] than group A. There were no significant differences in manual palpation score between autologous bone group (group A) and other groups (group B, C, and D). According to the results of microCT scan, the scores of group B and C were significantly lower than group A and there were no significant differences between other groups (D and E) and group A (Fig. 1). As a score of 5 or 6 was considered as indicating fusion in this study, mean value over 5 points was found only in group D and E whose β-TCP proportion was 70% with or without collagen. Mean histologic grades of groups B–E were significantly lower than group A.
To evaluate the effect of HA/β-TCP ratio, we performed intergroup comparison between group B and D, and C and E by post hoc analysis. As shown in Table 4, the score of manual palpation in group C was significantly higher than group E although no statistically significant difference was noted between group B and D. However, in terms of microCT analysis, group D showed significantly higher score than group B although there was no difference between group C and E (Fig. 1). There were no significant differences in histologic grade between groups.
To evaluate the effect of collagen on fusion, we performed intergroup comparison between group B and C, and D and E. Table 4 shows no significant differences in manual palpation scores. Regarding microCT analysis, the score was significantly higher in group C than group B although no significant difference was found between group D and E (Fig. 1). In terms of histologic grade, group C and E showed significantly higher score than group B and D.
At 12 weeks, bone volume on the fusion bed of group B and C showed no significant differences compared with group A, whereas group D and E had significantly lower fusion mass volume than group A (Table 5). Therefore, 3D reconstructive volumetric analysis indicated that 7:3 HA: β-TCP graft group (group B and C) had significantly higher fusion mass volume than autologous bone graft group and 3:7 HA: β-TCP graft group (group D and E) had significantly lower fusion mass volume than autologous bone graft group.
Analysis of the histological sections of each material was performed at 12 weeks after surgical procedure. As representative photos of each group are illustrated in Fig. 2, none of the grafted materials revealed a significant inflammatory reaction. Woven bone was identified around and close to the material in rat posterior laminar decorticated bone in all sections of fusion mass. The volume of the autologous tail bone in group A decreased progressively as bone formation increased at the periphery and within the block, leading to its virtual disappearance and almost complete closure of the cortex at 12 weeks. Also, in microCT scan of this fusion mass showed no distinction between graft and laminar bone (Fig. 2A). In group B with HA 70%, graft mass showed slower resorption than the other groups, thus the volume of fusion mass was larger than others as well. Histological results showed that changes to residual block content, peripheral bone resorption, new bone formation, and closure of the cortex after implantation were minimal in comparison with the other graft compositions (Fig. 2B). In 7:3 HA/β-TCP implanted groups (B and C), there was intermediate structural stability of fusion mass, and much less resorption than in 3:7 HA/β-TCP implanted groups (4 and 5). However, 3:7 HA/β-TCP implanted groups (D and E) revealed less distinctive features between graft and laminar bone and superior integration into recipient laminar bone, although much more resorbed fusion mass and reduced material volume were noted. According to histological comparison between groups with and without collagen (B versus C, D versus E), collagenous membrane which might be expected were found between materials and host bone. However, definite histological findings supportive of enhanced contact or incorporation could not be detected in this study.
Solid fusion is the most important prognostic factor for clinical success in fusion-based spinal surgery.36 Especially, geriatric population have more limitation for solid fusion even though autografts were used because they have more frequently diabetes, malnutrition and poor bone quality due to osteoporosis. Therefore, synthetic graft substitutes using various human BMP-2, β-TCP, HA, and collagen as an adjunct or a delivery system have been studied and customized as an alternative treatment for conventional autogenous bone grafting.3738
The present study was carried out to investigate the effect of different ratios of BCPs and collagen on posterior spinal fusion such as new bone formation, osteoinductivity, and biologic response between bone and material. Different ratios of HA/β-TCP with or without collagen could produce different biologic responses, thus ideal composition of two materials for various fusion situation should be determined for various situations.39 There was a difference in HA to β-TCP ratios between groups in this study; HA portion was 70% or 30%. Although β-TCP degraded rapidly after the graft, HA remained for a long time, and histologic findings revealed that most of them was encapsulated by fibrous tissue.3940 As previously reported, β-TCP is biodegradable and more easily resorbed than HA.41 In an ideal situation, it is better that a biodegradable bone substitute is slowly resorbed and replaced by natural bone.41 In cases that percentage of HA particle was excessive, new bone formation within graft materials might be partly inhibited.3940 Therefore, although appropriate BCP ratio was deemed important, it has yet to be determined.40 In previous studies, 20/80 was found to be an the optimal ratio of HA to β-TCP in the graft material for biodegradation and sufficient bone formation.40 On the contrary, Nery, et al.33 reported that 85:15 HA/β-TCP was the ideal ratio for new periodontal tissue attachment and bone regeneration. However, unlike calvarial bone defect or craniomaxillofacial bony area, spine segments which have to be fused are mobile during the fusion process. Thus, immediate posterior screw fixation is important for fusion stability. Our present rat fusion model included posterior spinal fusion without instrumentation to maintain mobility after implantation of bone. Thus, a stabilized, unreduced fusion mass by less resorbed HA might be needed in cases of fusion between separate vertebrae. We believe that this sets of our study are apart from previous research and results on bone filling effects.
In the present study, manual palpation score showed significantly lower in group E and the result of group D was also similar to group E, although not statistically proved. And group B and C showed similar strength of manual palpation compared to autologous bone graft (group A) BCP with 70% HA proportion which produced more structural stable fusion mass than 30% HA proportion. Early biodegradation and resorption of β-TCP could reduce the volume of graft material during the early phase of fusion process. However, microCT score and histologic grade of BCP with 3:7 HA/β-TCP ratio (group D and E) was higher than BCP with 7:3 HA/β-TCP ratio groups (group B and C). Especially, group D was significantly superior than group B in microCT analysis (5.5±0.8, 3.1±1.1, respectively, p=0.001), implying that bone-graft adherence and incorporation could be more superior in 3:7 HA/β-TCP ratio than 7:3 HA/β-TCP ratio.
The present study showed that collagen and BCP ceramic bone graft is as effective as autogenous graft in long bone fractures and traumatic osseous defects less than 30 mL in size and even in over 30 mL defect after tumor excision.42 Multicenter clinical trials have also shown collagen and BCP synthetic bone graft to be as effective as autogenous graft in long bone fractures.42 However, Muschler, et al.43 reported in 1996 that composites of purified bovine type I fibrillar skin collagen gel and granules of a BCP ceramic are ineffective as graft materials in this model when combined with autogenous cancellous bone or autogenous bone marrow. However, there were no significant differences between group B and C or group D and E in manual palpation score in this study. In 7:3 HA/β-TCP ratio intergroup comparison for collagen influence, the score of microCT of group C (collagen added) was significantly higher than group B (without collagen), and significant differences was found also between group D and E in 3:7 HA/β-TCP ratio intergroup comparison. The histologic grade of groups with collagen (group C and E) was higher than the score of groups without collagen (group B and D). Our present research showed a positive role of collagen for posterior lumbar spinal fusion, these results being expected based on previous results: the majority of previous studies investigated the influence of collagen membrane wrapping on bone fusion, since the wrapping, allows osteoblasts to enter for bone formation enhancement and barrier for fibrous tissue growing into bony defect.2444 We can speculate that collagen added to BCP allows for a relatively smoother texture to which mobile segments adhere and to act as a barrier for inhibiting fibroblast migration into the BCP, while allowing osteoblast migration.
Our research has several limitations. First, comparison by manual palpation score with unblinded fashion is obviously inferior to biomechanical bending test by machine. Thus, we tried to exclude examiners subjective feelings by summation of multiple scores and binary simplification of score system. Second, inconsistencies in the surgical method have biased fusion outcomes. We focused on uneven sizes of the transverse process among rats and decided to use posterior laminar surface for graft bed.
In spite of a few shortcomings, we found that a 7:3 HA/β-TCP ratio with collagen augmentation, rather than a 3:7 HA/β-TCP ratio, could be a more favorable graft substitute for lumbar spinal fusion, as an appropriate HA proportion is needed for initial stabilization of the fusion mass. In our study, we noted a positive role for collagen as an adjunct for spinal bone fusion. Further studies are needed to determine the exact effect of collagen on spinal bone fusion.
Figures and Tables
 | Fig. 1Representative microcomputed tomography axial and sagittal view sections of fusion masses from each group. (A) Autobone (group A) showed complete union without graft-bone interface. (B) 7:3 HA: β-TCP (group B) revealed relatively larger fusion mass but less incorporated into recipient lamina. (C) 7:3 HA: β-TCP+collagen (group C) showed little incorporation into lamina but more remaining fusion mass. (D) 3:7 HA: β-TCP (group D) showed reduced fusion mass but revealed good graft incorporation into recipient lamina. (E) 3:7 HA: β-TCP+collagen (group E) demonstrated excellent graft incorporation into recipient lamina. HA, hydroxyapatite; β-TCP, beta tricalcium phosphate. |
 | Fig. 2Representative histologic features of fusion mass on goldner's trichrome staining. (A) Immature bone with narrow trabecular was formed along the cutting surface of bone defect (group A). (B) Peripheral bone resorption, new bone formation, and closure of cortex were minimal in comparison with other group (group B), (C) intermediate structural stability of fusion mass but less resorption than group D and E (group C). (D) Less distinctive features between graft and laminar bone and superior integration into recipient bone were shown, and reduced volume of fusion mass was also noted (group D). (E) There was excellent incorporation of graft into laminar bone but much less volume of fusion mass due to excessive resorption (group E). |
Table 1
Detailed Descriptions of Group

Table 2
Histologic Grade

Table 3
Overall Results of Each Groups for Manual Palpation, MicroCT, and Histologic Grade

Table 4
Results of Statistical Analysis
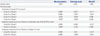
Table 5
Total Volume of Fusion Mass in Each Group (12 Weeks after Surgery)

ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for (6-2011-0065).
References
1. Zurbriggen C, Markwalder TM, Wyss S. Long-term results in patients treated with posterior instrumentation and fusion for degenerative scoliosis of the lumbar spine. Acta Neurochir (Wien). 1999; 141:21–26.

2. Gertzbein SD, Hollopeter M, Hall SD. Analysis of circumferential lumbar fusion outcome in the treatment of degenerative disc disease of the lumbar spine. J Spinal Disord. 1998; 11:472–478.
3. Rompe JD, Eysel P, Hopf C. Clinical efficacy of pedicle instrumentation and posterolateral fusion in the symptomatic degenerative lumbar spine. Eur Spine J. 1995; 4:231–237.

4. Herkowitz HN, Sidhu KS. Lumbar spine fusion in the treatment of degenerative conditions: current indications and recommendations. J Am Acad Orthop Surg. 1995; 3:123–135.

5. Flouzat-Lachaniette CH, Ghazanfari A, Bouthors C, Poignard A, Hernigou P, Allain J. Bone union rate with recombinant human bone morphogenic protein-2 versus autologous iliac bone in PEEK cages for anterior lumbar interbody fusion. Int Orthop. 2014; 38:2001–2007.

6. Kim DH, Rhim R, Li L, Martha J, Swaim BH, Banco RJ, et al. Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J. 2009; 9:886–892.

7. Al-Sayyad MJ, Abdulmajeed TM. Fracture of the anterior iliac crest following autogenous bone grafting. Saudi Med J. 2006; 27:254–258.
8. Pitzen T, Kränzlein K, Steudel WI, Strowitzki M. [Complaints and findings at the iliac crest donor site following anterior cervical fusion]. Zentralbl Neurochir. 2004; 65:7–12.
9. Delawi D, Dhert WJ, Castelein RM, Verbout AJ, Oner FC. The incidence of donor site pain after bone graft harvesting from the posterior iliac crest may be overestimated: a study on spine fracture patients. Spine (Phila Pa 1976). 2007; 32:1865–1868.

10. Ito Z, Imagama S, Kanemura T, Hachiya Y, Miura Y, Kamiya M, et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion (PLIF): a multicenter study. Eur Spine J. 2013; 22:1158–1163.

11. Lippman CR, Salehi SA, Liu JC, Ondra SL. Salvage technique of posterior iliac bolt placement in long-segment spinal constructs with a previous posterior iliac crest harvest: technical note. Neurosurgery. 2006; 58:1 Suppl. ONS–E178.

12. Emery SE, Hughes SS, Junglas WA, Herrington SJ, Pathria MN. The fate of anterior vertebral bone grafts in patients irradiated for neoplasm. Clin Orthop Relat Res. 1994; (300):207–212.

13. Taghavi CE, Lee KB, Keorochana G, Tzeng ST, Yoo JH, Wang JC. Bone morphogenetic protein-2 and bone marrow aspirate with allograft as alternatives to autograft in instrumented revision posterolateral lumbar spinal fusion: a minimum two-year follow-up study. Spine (Phila Pa 1976). 2010; 35:1144–1150.

14. Ajiboye RM, Hamamoto JT, Eckardt MA, Wang JC. Clinical and radiographic outcomes of concentrated bone marrow aspirate with allograft and demineralized bone matrix for posterolateral and interbody lumbar fusion in elderly patients. Eur Spine J. 2015; 24:2567–2572.

15. Virk S, Sandhu HS, Khan SN. Cost effectiveness analysis of graft options in spinal fusion surgery using a Markov model. J Spinal Disord Tech. 2012; 25:E204–E210.

16. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012; 132:1105–1110.

17. Thalgott JS, Giuffre JM, Fritts K, Timlin M, Klezl Z. Instrumented posterolateral lumbar fusion using coralline hydroxyapatite with or without demineralized bone matrix, as an adjunct to autologous bone. Spine J. 2001; 1:131–137.

18. Martin S, Daculsi G, Passuti N, Le Nihouannen JC, Lajat Y, Resche F. [Bone integration within calcium phosphate ceramic by creation of posterior inter-articular lumbar spine fusions in dogs]. Neurochirurgie. 1989; 35:209–215.
19. Yi J, Lee GW, Nam WD, Han KY, Kim MH, Kang JW, et al. A prospective randomized clinical trial comparing bone union rate following anterior cervical discectomy and fusion using a polyetheretherketone cage: hydroxyapatite/B-tricalcium phosphate mixture versus hydroxyapatite/demineralized bone matrix mixture. Asian Spine J. 2015; 9:30–38.

20. Sugawara T, Itoh Y, Hirano Y, Higashiyama N, Mizoi K. β-tricalcium phosphate promotes bony fusion after anterior cervical discectomy and fusion using titanium cages. Spine (Phila Pa 1976). 2011; 36:E1509–E1514.

21. Cosar M, Ozer AF, Iplikcioglu AC, Oktenoglu T, Kosdere S, Sasani M, et al. The results of beta-tricalcium phosphate coated hydroxyapatite (beta-TCP/HA) grafts for interbody fusion after anterior cervical discectomy. J Spinal Disord Tech. 2008; 21:436–441.

22. Miron RJ, Sculean A, Shuang Y, Bosshardt DD, Gruber R, Buser D, et al. Osteoinductive potential of a novel biphasic calcium phosphate bone graft in comparison with autographs, xenografts, and DFDBA. Clin Oral Implants Res. 2016; 27:668–675.

23. Lobo SE, Glickman R, da Silva WN, Arinzeh TL, Kerkis I. Response of stem cells from different origins to biphasic calcium phosphate bioceramics. Cell Tissue Res. 2015; 361:477–495.

24. Calvo-Guirado JL, Ramírez-Fernández MP, Delgado-Ruíz RA, Maté-Sánchez JE, Velasquez P, de Aza PN. Influence of biphasic β-TCP with and without the use of collagen membranes on bone healing of surgically critical size defects. A radiological, histological, and histomorphometric study. Clin Oral Implants Res. 2014; 25:1228–1238.

25. Lim HC, Zhang ML, Lee JS, Jung UW, Choi SH. Effect of different hydroxyapatite: β-tricalcium phosphate ratios on the osteoconductivity of biphasic calcium phosphate in the rabbit sinus model. Int J Oral Maxillofac Implants. 2015; 30:65–72.

26. Ebrahimi M, Pripatnanont P, Suttapreyasri S, Monmaturapoj N. In vitro biocompatibility analysis of novel nano-biphasic calcium phosphate scaffolds in different composition ratios. J Biomed Mater Res B Appl Biomater. 2014; 102:52–61.

27. Nevins M, Nevins ML, Schupbach P, Kim SW, Lin Z, Kim DM. A prospective, randomized controlled preclinical trial to evaluate different formulations of biphasic calcium phosphate in combination with a hydroxyapatite collagen membrane to reconstruct deficient alveolar ridges. J Oral Implantol. 2013; 39:133–139.

28. Ebrahimi M, Pripatnanont P, Monmaturapoj N, Suttapreyasri S. Fabrication and characterization of novel nano hydroxyapatite/β-tricalcium phosphate scaffolds in three different composition ratios. J Biomed Mater Res A. 2012; 100:2260–2268.

29. Jensen SS, Bornstein MM, Dard M, Bosshardt DD, Buser D. Comparative study of biphasic calcium phosphates with different HA/TCP ratios in mandibular bone defects. A long-term histomorphometric study in minipigs. J Biomed Mater Res B Appl Biomater. 2009; 90:171–181.

30. Friedmann A, Dard M, Kleber BM, Bernimoulin JP, Bosshardt DD. Ridge augmentation and maxillary sinus grafting with a biphasic calcium phosphate: histologic and histomorphometric observations. Clin Oral Implants Res. 2009; 20:708–714.

31. LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med. 2003; 14:201–209.
32. Bouler JM, LeGeros RZ, Daculsi G. Biphasic calcium phosphates: influence of three synthesis parameters on the HA/beta-TCP ratio. J Biomed Mater Res. 2000; 51:680–684.

33. Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol. 1992; 63:729–735.

34. Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman JR. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004; (86-A):2243–2250.
35. Biswas D, Bible JE, Whang PH, Miller CP, Jaw R, Miller S, et al. Augmented demineralized bone matrix: a potential alternative for posterolateral lumbar spinal fusion. Am J Orthop (Belle Mead NJ). 2010; 39:531–538.
36. Kim JH, Park JY, Yi S, Kim KH, Kuh SU, Chin DK, et al. Anterior cervical discectomy and fusion alters whole-spine sagittal alignment. Yonsei Med J. 2015; 56:1060–1070.

37. Drespe IH, Polzhofer GK, Turner AS, Grauer JN. Animal models for spinal fusion. Spine J. 2005; 5:6 Suppl. 209S–216S.

38. Bostan B, Güneş T, Aşçı M, Sen C, Keles¸temur MH, Erdem M, et al. Simvastatin improves spinal fusion in rats. Acta Orthop Traumatol Turc. 2011; 45:270–275.

39. Maté-Sánchez de Val JE, Mazón P, Guirado JL, Ruiz RA, Ramírez Fernández MP, Negri B, et al. Comparison of three hydroxyapatite/ β-tricalcium phosphate/collagen ceramic scaffolds: an in vivo study. J Biomed Mater Res A. 2014; 102:1037–1046.

40. Yun PY, Kim YK, Jeong KI, Park JC, Choi YJ. Influence of bone morphogenetic protein and proportion of hydroxyapatite on new bone formation in biphasic calcium phosphate graft: two pilot studies in animal bony defect model. J Craniomaxillofac Surg. 2014; 42:1909–1917.

41. Eftekhari H, Farahpour MR, Rabiee SM. Histopathological evaluation of potential impact of β-tricalcium phosphate (HA+ β-TCP) granules on healing of segmental femur bone defect. Bratisl Lek Listy. 2015; 116:30–34.

42. Grimes JS, Bocklage TJ, Pitcher JD. Collagen and biphasic calcium phosphate bone graft in large osseous defects. Orthopedics. 2006; 29:145–148.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download