Abstract
Purpose
Distinguishing infiltrative renal cell carcinoma (RCC) from transitional cell carcinoma (TCC) is a challenging issue due to their radiologic similarities. We evaluated systemic inflammatory biomarkers as parameters for distinguishing tumor types.
Materials and Methods
A computerized search of medical records from November 2005 to October 2015 identified 116 patients with infiltrative renal masses who were difficult to diagnose confirmatively in radiological study. We investigated the diagnostic efficacy among these patients with their preoperative absolute neutrophil counts (ANC), absolute lymphocyte counts (ALC), absolute monocyte counts (AMC), neutrophil-lymphocyte ratio (NLR), and lymphocyte-monocyte ratio (LMR).
Results
The infiltrative RCC group demonstrated significantly lower ALC {1449/µL (1140–1896), median [interquartile range (IQR)]} than the TCC group [1860/µL (1433–2342), p=0.016]. LMR [median (IQR)] also was lower in the infiltrative RCC group [2.98 (2.32–4.14) vs. TCC group 4.10 (2.86–6.09); p=0.011]. In subgroup analysis, non-metastatic infiltrative RCC showed lower ALC and LMR and higher NLR than non-metastatic TCC. Within non-metastatic infiltrative renal masses, multivariate logistic regression analysis revealed that younger patient age and lower LMR were associated with infiltrative RCC [odds ratios (OR) 0.874, p=0.024 and OR 0.461, p=0.048, respectively]. Receiver operating characteristic curve analysis showed that younger age and lower LMR were highly predictive of non-metastatic RCC (area under the curve=0.919, p<0.001).
Clinicians frequently encounter infiltrative renal masses in their practices. These lesions lack a sharp border of demarcation with the normal parenchyma, showing ill-defined zones of transition between the lesion and normal parenchyma.1 The masses represent a number of pathologies, such as infiltrative renal cell carcinoma (RCC), transitional cell carcinoma (TCC), metastatic cancer, medullary carcinoma, renal sarcoma, lymphoma, and inflammatory diseases.2 However, due to radiologic similarities among these conditions, multi-detector computed tomography (MDCT) is not helpful in distinguishing between lesion types. Distinguishing infiltrative RCC from TCC is a particularly critical process, because of differences in surgical treatment: nephrectomy is carried out in cases of RCC, while nephroureterectomy with lymphadenectomy is usually performed in TCC.134
RCC is the most common type of kidney parenchymal cancer, accounting for 85% to 90% of cases.4 About 6% of RCCs manifest as infiltrative lesions.1 Meanwhile, TCC of the renal pelvis usually displays an infiltrative growth pattern.2 Both infiltrative RCC and TCC show up as poorly marginated areas of diminished enhancement in MDCT.1 A few studies have tried to use CT to differentiate between the two cancers. Raza, et al.2 reported six representative CT findings that could be used to distinguish centrally located RCC from intrarenal TCC. Bata, et al.3 reported an additional parameter using attenuation ratios in different phases. Nevertheless, Li, et al.5 refuted these two reports, reporting that imaging findings of hypovascular RCC are indistinguishable from TCC, and that the clinical application of CT in this area is still not acceptable for confirmative diagnoses.
Since RCC is an immunologic cancer,6 our study focused on the altered immunology that is characteristic of RCC pathogenesis. Several serum biomarkers and hematological indices representative of inflammatory response, including C reactive protein, fibrinogen, absolute monocyte count (AMC), absolute neutrophil count (ANC),7 absolute lymphocyte count (ALC),68 lymphocyte-monocyte ratio (LMR),910 neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio,111213141516171819 have been investigated as biomarkers to predict prognosis, oncologic outcomes, and treatment responses in RCC patients.202122 Herein, we hypothesized that systemic inflammatory biomarkers could play a major role in distinguishing infiltrative RCC from TCC and attempted to investigate their accuracy in differential diagnosis of patients with infiltrative renal masses.
The study was performed in accordance with applicable laws and regulations, good clinical practices, and ethical principles as described in the Declaration of Helsinki. Severance Hospital Institutional Review Board approved this study protocol (Approval number: 4-2016-0021).
We retrospectively reviewed a database of RCC and TCC patients whose diagnoses were not confirmed in preoperative MDCT imaging. A total of 117 patients from November 2005 to October 2015 with typically infiltrative renal masses were further assessed. They were confirmatively diagnosed by either surgical resection or percutaneous needle biopsy. From this initial cohort, 25 patients were excluded due to inability to undergo pathological diagnosis for personal reason or incomplete preoperative blood test or other pathologic findings, such as 1) acute pyelonephritis (n=5), 2) spindle cell sarcoma (n=3), 3) other primary cancer metastasis (n=3), and 4) oncocytoma (n=1). Patients with relevant comorbidities affecting systemic inflammatory response markers, such as chronic liver disease, immunosuppression, cytotoxic medication, hemato-oncological disease, autoimmune disease, or acute infection status, were also excluded (n=4). Finally, 88 patients with pathologically proven RCC or TCC that showed an infiltrative growth pattern in MDCT were included in our study (Fig. 1).
Blood samples were collected in calcium ethylenediaminetetraacetic acid tubes, and an auto-analyzer (XN-9000-Hematology Analyzer, Sysmex, IL, USA) was used to evaluate ANC, ALC, AMC, NLR, and LMR as systemic inflammatory biomarkers. Blood counts were measured within 1 month prior to surgery.
As described in detail by Keskin, et al.,11 all of the patients were examined using a 64-multidetector CT scanner (Siemens SOMATOM Sensation 64, Erlangen, Germany) set for 0.6-mm collimation, 3-mm slice thickness, 3-mm increments, 100 kV, 135 mAs, pitch of 0.9, and a gantry rotation time of 0.33 s. A scout image was obtained first, followed by an unenhanced phase, corticomedullary phase (25 seconds after contrast injection), nephrographic phase (60 seconds after contrast injection), and excretory phase (5 minutes after contrast injection), sequentially. All patients received 100 mL of nonionic contrast medium (Ultravist 300; Bayer Schering Pharma, Berlin, Germany) with a flow rate of 5 mm/sec.
Kolmogorov-Smirnov and Shapiro-Wilk normality tests were used to verify the normal distribution of continuous variables. The differences between two groups for normally distributed variables were tested using an independent Student's t-test, while the Mann-Whitney U test was used to compare non-parametrically distributed variables. Normally distributed variables are expressed as median±SD, and non-parametrically distributed variables are expressed as median (IQR). The differences between the categorical variables were determined by a χ2-test.
Univariate and multivariate logistic regression analyses were performed to determine correlations with age, sex, tumor size, and systemic inflammatory biomarkers. In a multivariate model, significant variables in univariate analysis were taken into account. Odds ratios estimated from the logistic analyses are reported as relative risks with corresponding 95% confidence intervals (CIs).
Receiver operating characteristic (ROC) curve analysis was subsequently performed using the multivariable model. The area under the curve (AUC) was calculated. All statistical analyses were performed using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). All p-values <0.05 were considered statistically significant, and all statistical tests were two-sided.
A total of 63 patients with infiltrative RCC and 25 patients with TCC were enrolled in our study. RCC patients were significantly younger than TCC patients (55.4±13.6 years vs. 69.7±10.2 years, p<0.001). Tumor size tended to be larger in the RCC group, with a mean size of 7.6±4.1 cm, compared to 6.1±3.1 cm for the TCC group; however, the difference was not statistically significant. Fewer than half of the patients in both groups showed nodal metastasis (Table 1). Likewise, there were more non-metastatic diseases than metastatic diseases. Infiltrative RCC patients tended to have high Fuhrman grades with clear cell dominant features.
ANC, AMC, and NLR did not statistically differ between the two groups (Table 2). ALC was significantly lower for the RCC group than the TCC group [1499/µL (1140, 1896) vs. 1860/µL (1433, 2342), p=0.016]. Likewise, LMR was significantly lower in the RCC group than in the TCC group [2.98 (2.32, 4.14) vs. 4.10 (2.86, 6.09), p=0.011].
In subgroup analyses, ALC, NLR, and LMR significantly differed between the two groups in N0M0 renal masses (Table 3). In particular, ALC and LMR were useful markers for distinguishing small RCC and TCC tumors (≤ 4 cm) (p-values=0.020 and 0.003, respectively). Conversely, no inflammatory biomarker was useful in distinguishing metastatic RCC from metastatic TCC or in classifying a renal mass larger than 4 cm.
When we performed logistic regression within the non-metastatic group, young age at diagnosis, low ALC, and low LMR were significantly associated with RCC instead of TCC in univariate analysis (Table 4). In multivariate analysis, age at diagnosis and LMR were the only two variables that were statistically significant differentiators of RCC and TCC, with young age and low LMR correlating with higher probability of RCC than TCC. The odds ratio (95% CI) for age at diagnosis was 0.874 (0.778–0.982; p=0.024); for LMR it was 0.461 (0.214–0.994; p=0.048). We subsequently performed ROC curve analysis with probabilities extracted from the logistic regression, and the AUC was 0.919 (p<0.001), indicating strong correlations between RCC and younger age or LMR (date not shown). For the small renal mass (≤4 cm) group, however, multivariate logistic regression analysis revealed no significant correlations with any parameters (Supplementary Table 1, only online).
About 6% of RCC cases involve infiltration of surrounding tissues, appearing irregular and with ill-defined margins on MDCT. For this reason, in suspected RCC cases, MDCT is technically limited for excluding other infiltrative diseases and defining the correct diagnosis. Our study demonstrated the clinical usefulness of inflammatory biomarkers in distinguishing infiltrative RCC from TCC, which are the two most common infiltrative renal diseases. The usefulness of these biomarkers is already established in many other fields. These markers are widely used as prognostic factors not only for acute and chronic infections, but also for cancerous and non-cancerous inflammatory diseases. Because inflammatory biomarker evaluation is relatively cheap and blood samples are easy to access, these parameters are important to consider as diagnostic tools.
Cancer has recently been considered to be as a disease of chronic inflammation. This classification is based on the pathological presence of inflammatory cells and its related mediators, such as cytokines in tumor tissues. Furthermore, according to Mantovani, et al.,23 tissue remodeling and angiogenesis processes in cancers are similar to those seen in chronic inflammatory responses and tissue repair. Additionally, Mantovani, et al.24 demonstrated that immature myeloid cells play a key role in both chronic infection and tumor microenvironment.
RCC is one of the most well-known cancers associated with a pathogenesis that includes altered immune activity. Several studies demonstrate poorer prognosis in RCC patients with higher monocyte and neutrophils levels and lower lymphocyte counts.7 According to Frankenberger, et al.,25 growing RCC tumors influence the activity of effector lymphocytes and modulate the composition of immune infiltrates. As the disease progresses, peripheral circulation are also affected as an increase in the number of circulating myeloid cells and regulatory T cells.
There are several reports that neutrophils and macrophages play major roles in RCC tumor progression. The level of circulating monocytes can reflect the formation or presence of tumor-associated macrophages. Many macrophage-released soluble factors directly stimulate the growth of tumor cells and promote tumor cell migration and metastasis.9 Donskov, et al.7 reported that among macrophage and neutrophil products, ROS may not only induce genomic instability, but also damage antitumor immune effector cells. Another study demonstrated that co-cultivation of tumor cells with macrophages leads to enhanced invasiveness of the malignant cells by TNF-α-dependent MMP induction in the macrophages.24 Given these laboratory findings, clinical researchers have focused on the role of inflammatory biomarkers, especially monocyte and lymphocyte counts, in peripheral circulation as oncologic outcome predictors or prognostic factors in RCC patients. Based on these above findings, the LMR has been assessed as a good candidate inflammatory biomarker for RCC. Several studies focused on the value of preoperative and postoperative LMR as an important prognostic factor in metastatic and non-metastatic RCC patients.926272829
Additionally, there are several reports of a predictive role for LMR in upper urinary tract TCC. Elevated preoperative LMR also has prognostic value in non-metastatic upper urinary tract TCC.30 Furthermore, the presence of neutrophilia with relative lymphocytopenia predicts a worse oncological prognosis in patients with localized upper urinary tract TCC.313233
As a novel perspective, we focused on the possibility that inflammatory biomarkers could distinguish RCC from other infiltrative renal masses, especially TCC that mimics RCC on diagnostic images. We hypothesized that infiltrative RCC and TCC might demonstrate different degrees of biomarker changes due to differences in tumor biology. Of the 63 pathologically-proven RCC patients in our study, more than half (35/63, 55.6%) required renal biopsy (20/63, 31.7%) or diagnostic ureterorenoscopy (15/63, 23.8%). Eight (12.7%) cases of pathologically-proven RCC underwent nephroureterectomy instead of nephrectomy, due to relatively low suspicion of RCC in preoperative MDCT. Likewise, of the 25 pathologically-proven TCC patients, 15 (60%) underwent renal biopsy or diagnostic ureterorenoscopy preoperatively. Six patients (24%) underwent nephrectomy instead of nephroureterectomy, and consequently required subsequent remnant-ureterectomy surgery. Overall, about 16% of infiltrative renal masses in our cohort were misdiagnosed preoperatively, and underwent inappropriate surgery. Adding cytology results also did not help. Among 25 pathologically-proven TCC patients, cytology was performed in 16 cases. Only 2 cases out of 16 (12.5%) showed positive results for TCC.
Previous studies report that as RCC tumor burden grows, the values of NLR and LMR increase and decrease, respectively. We performed subgroup analysis to see if the biomarker parameters could be diagnostically relevant in cases with relatively lower tumor burden. We especially focused on the clinical power of inflammatory biomarkers as tools for diagnosis in surgical candidates without metastasis. In the non-metastatic group, we identified ALC, NLR, and LMR as potential biomarkers that distinguished infiltrative RCC from TCC. Logistic regression analysis revealed that LMR is the only significant useful inflammatory biomarker for distinguishing RCC from TCC. In the ROC curve analysis, the AUC was 0.817 (p-value=0.002), and the cut off value was 5.250 (data not shown). This value is slightly higher than the already mentioned values ranging from 3.0–5.2 as an independent prognostic factor. Furthermore, in patients with small renal masses less than 4 cm, we confirmed that ALC and LMR could be helpful in distinguishing RCC from TCC by Mann-Whitney U tests (Table 3), although multivariate regression showed no statistically significant biomarkers. With the help of these biomarkers, we may avoid choosing the wrong surgical method in planning resections; moreover, we may be able to recommend additional preoperative renal biopsies or diagnostic ureterorenoscopies in cases of mismatch between imaging results and biomarker results. Nevertheless, the inflammatory biomarkers were less effective in distinguishing infiltrative large (>4 cm) or metastatic cancers. We think this result originated from the common biologic characteristics of metastatic solid cancers. According to the results of a meta-analysis performed by Teng, et al.,34 increased pretreatment LMR is correlated with significantly favorable outcomes in patients with general solid tumors. Although the exact mechanism of the prognostic value of LMR in solid tumor is poorly understood, we could infer that the effect of cancer-related inflammation could be maximized for general advanced solid cancers with metastasis, obscuring the role of inflammatory biomarkers in distinguishing cancer types.
This study focused primarily on non-metastatic infiltrative renal masses. For patients with lesions of this nature, we typically consider surgical resection regardless of the exact pathology. By simple calculations based on a basic blood workup, urologists can gain insight into the type of infiltrative lesion for designing surgical plans in preoperative settings.
The current study has several limitations. First, although we attempted to control for possible confounding factors that could lead to biased results, this was a retrospective study with a relatively small number of patients. Another potential limitation is that we did not compare serum LMR with the extent of inflammatory cell infiltration within and surrounding tumor tissue. Such histological correlation should be considered in future analyses. However, even considering these limitations, we believe our study outlines the clinically benefits of the noted biomarkers in distinguishing infiltrative RCC from TCC.
In conclusion, inflammatory biomarkers could be helpful in distinguishing infiltrative renal masses. Younger age and lower LMR are more likely to imply infiltrative RCC rather than TCC. Using these inflammatory biomarkers in conjunction with radiological imaging, non-metastatic infiltrative RCC could be diagnosed with greater certainty.
Figures and Tables
Fig. 1
(A and B) Pathologically proven infiltrative renal cell carcinoma and transitional cell carcinoma in preoperative multi-detector computed tomography. (C and D) Cross sections of the pathologic specimens of each tumor after surgical resection.
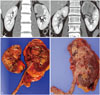
Table 1
Characteristics of Patients with Infiltrating Renal Cell Carcinomas (RCC) and RCC-Mimicking Transitional Cell Carcinomas (TCC)

Table 2
Inflammatory Biomarker Measurements in RCC and TCC

Table 3
Inflammatory Biomarker Associations with Pathological Parameters in Subgroups of Infiltrative RCC and TCC

Table 4
Univariate and Multivariate Analysis of Correlations between Demographic and Blood Count Variables with Non-Metastatic Infiltrative Renal Cell Carcinoma

References
1. Pickhardt PJ, Lonergan GJ, Davis CJ Jr, Kashitani N, Wagner BJ. From the archives of the AFIP. Infiltrative renal lesions: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics. 2000; 20:215–243.
2. Raza SA, Sohaib SA, Sahdev A, Bharwani N, Heenan S, Verma H, et al. Centrally infiltrating renal masses on CT: differentiating intrarenal transitional cell carcinoma from centrally located renal cell carcinoma. AJR Am J Roentgenol. 2012; 198:846–853.

3. Bata P, Tarnoki DL, Tarnoki AD, Novak PK, Gyebnar J, Kekesi D, et al. Transitional cell and clear cell renal carcinoma: differentiation of distinct histological types with multiphase CT. Acta Radiol. 2014; 55:1112–1119.

4. Sheir KZ, El-Azab M, Mosbah A, El-Baz M, Shaaban AA. Differentiation of renal cell carcinoma subtypes by multislice computerized tomography. J Urol. 2005; 174:451–455.

5. Li Y, Ding YU, Chen D, Yu Z, Gui Y, Yang S, et al. Renal cell carcinoma growing into the renal pelvis and mimicking transitional cell carcinoma: a case report and literature review. Oncol Lett. 2015; 9:1869–1872.

6. Saroha S, Uzzo RG, Plimack ER, Ruth K, Al-Saleem T. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol. 2013; 189:454–461.

7. Donskov F, Hokland M, Marcussen N, Torp Madsen HH, von der Maase H. Monocytes and neutrophils as ‘bad guys’ for the outcome of interleukin-2 with and without histamine in metastatic renal cell carcinoma--results from a randomised phase II trial. Br J Cancer. 2006; 94:218–226.

8. Mehrazin R, Uzzo RG, Kutikov A, Ruth K, Tomaszewski JJ, Dulaimi E, et al. Lymphopenia is an independent predictor of inferior outcome in papillary renal cell carcinoma. Urol Oncol. 2015; 33:388.e19–388.e25.

9. Hutterer GC, Stoeckigt C, Stojakovic T, Jesche J, Eberhard K, Pummer K, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol. 2014; 32:1041–1048.

10. Park YH, Ku JH, Kwak C, Kim HH. Post-treatment neutrophil-to-lymphocyte ratio in predicting prognosis in patients with metastatic clear cell renal cell carcinoma receiving sunitinib as first line therapy. Springerplus. 2014; 3:243.

11. Keskin S, Keskin Z, Taskapu HH, Kalkan H, Kaynar M, Poyraz N, et al. Prognostic value of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios, and multiphasic renal tomography findings in histological subtypes of renal cell carcinoma. BMC Urol. 2014; 14:95.

12. Hu K, Lou L, Ye J, Zhang S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open. 2015; 5:e006404.

13. Wen RM, Zhang YJ, Ma S, Xu YL, Chen YS, Li HL, et al. Preoperative neutrophil to lymphocyte ratio as a prognostic factor in patients with non-metastatic renal cell carcinoma. Asian Pac J Cancer Prev. 2015; 16:3703–3708.

14. Pichler M, Hutterer GC, Stoeckigt C, Chromecki TF, Stojakovic T, Golbeck S, et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer. 2013; 108:901–907.

15. Santoni M, De Giorgi U, Iacovelli R, Conti A, Burattini L, Rossi L, et al. Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Br J Cancer. 2013; 109:1755–1759.

16. Ohno Y, Nakashima J, Ohori M, Hatano T, Tachibana M. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010; 184:873–878.

17. Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012; 187:411–417.

18. de Martino M, Pantuck AJ, Hofbauer S, Waldert M, Shariat SF, Belldegrun AS, et al. Prognostic impact of preoperative neutrophil-to-lymphocyte ratio in localized nonclear cell renal cell carcinoma. J Urol. 2013; 190:1999–2004.

19. Viers BR, Houston Thompson R, Boorjian SA, Lohse CM, Leibovich BC, Tollefson MK. Preoperative neutrophil-lymphocyte ratio predicts death among patients with localized clear cell renal carcinoma undergoing nephrectomy. Urol Oncol. 2014; 32:1277–1284.

20. Riemann D, Hase S, Fischer K, Seliger B. Granulocyte-to-dendritic cell-ratio as marker for the immune monitoring in patients with renal cell carcinoma. Clin Transl Med. 2014; 3:13.

21. Fox P, Hudson M, Brown C, Lord S, Gebski V, De Souza P, et al. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer. 2013; 109:147–153.

22. Lucca I, de Martino M, Hofbauer SL, Zamani N, Shariat SF, Klatte T. Comparison of the prognostic value of pretreatment measurements of systemic inflammatory response in patients undergoing curative resection of clear cell renal cell carcinoma. World J Urol. 2015; 33:2045–2052.

23. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–444.

24. Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006; 25:315–322.

25. Frankenberger B, Noessner E, Schendel DJ. Immune suppression in renal cell carcinoma. Semin Cancer Biol. 2007; 17:330–343.

26. Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015; 113:626–633.

27. Chang Y, Fu Q, Xu L, Zhou L, Liu Z, Yang Y, et al. Prognostic value of preoperative lymphocyte to monocyte ratio in patients with nonmetastatic clear cell renal cell carcinoma. Tumour Biol. 2016; 37:4613–4620.

28. Gu L, Ma X, Wang L, Li H, Chen L, Li X, et al. Prognostic value of a systemic inflammatory response index in metastatic renal cell carcinoma and construction of a predictive model. Oncotarget. 2016; 07. 16. [Epub]. DOI: 10.18632/oncotarget.10626.

29. Xia WK, Wu X, Yu TH, Wu Y, Yao XJ, Hu H. Prognostic significance of lymphocyte-to-monocyte ratio and CRP in patients with nonmetastatic clear cell renal cell carcinoma: a retrospective multicenter analysis. Onco Targets Ther. 2016; 9:2759–2767.
30. Hutterer GC, Sobolev N, Ehrlich GC, Gutschi T, Stojakovic T, Mannweiler S, et al. Pretreatment lymphocyte-monocyte ratio as a potential prognostic factor in a cohort of patients with upper tract urothelial carcinoma. J Clin Pathol. 2015; 68:351–355.

31. Luo HL, Chen YT, Chuang YC, Cheng YT, Lee WC, Kang CH, et al. Subclassification of upper urinary tract urothelial carcinoma by the neutrophil-to-lymphocyte ratio (NLR) improves prediction of oncological outcome. BJU Int. 2014; 113:E144–E149.

32. Dalpiaz O, Ehrlich GC, Mannweiler S, Hernández JM, Gerger A, Stojakovic T, et al. Validation of pretreatment neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. BJU Int. 2014; 114:334–339.

33. Dalpiaz O, Pichler M, Mannweiler S, Martín Hernández JM, Stojakovic T, Pummer K, et al. Validation of the pretreatment derived neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. Br J Cancer. 2014; 110:2531–2536.

34. Teng JJ, Zhang J, Zhang TY, Zhang S, Li BS. Prognostic value of peripheral blood lymphocyte-to-monocyte ratio in patients with solid tumors: a meta-analysis. Onco Targets Ther. 2016; 9:37–47.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download