Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1The flow chart on the enrollment processes for study participants. *This was defined as the fulfillment of all EGDT components and achievement of the final goal of superior vena cava oxygen saturation ≥70% within 6 hrs after arrival at the emergency department, as well as faithfully implemented EGDT and initial resuscitation of Surviving Sepsis Campaign international guidelines for management of severe sepsis and septic shock according to the recent update version at enrollment. EGDT, early goal-directed therapy. |
 | Fig. 2Changing pattern of PTX3 levels at HD 0, 3, and 7 in survivors and non-survivors. The middle and upper/lower error bars mean the median and interquartile ranges, respectively. PTX3, pentraxin 3; HD, hospital day. |
 | Fig. 3Comparison of ROC curves for inflammatory markers (A) and APACH II/SOFA score (B) measured at hospital day 0 for the prediction of 28-day all-cause mortality. PTX3, delta neutrophil index, CRP and procalcitonin were measured at hospital day 0 within 24 hrs of arrival at the emergency department. SOFA score was assessed upon arrival at the emergency department and APACHE II score was assessed based on the worst values over the first 24 hrs of intensive care unit care. ROC, receiver operating characteristic; AUC, area under the ROC curve; CI, confidential interval; PTX3, pentraxin 3; PCT, procalcitonin; CRP, C-reactive protein; DNI, delta neutrophil index; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment. |
 | Fig. 4Kaplan-Meier survival curve according to PTX3 level on hospital day 0 with a cut-off point of 140 ng/mL (A) and comparison of short-term change in PTX3 level between hospital day 0 and 3 in both survivors and non-survivors (B). PTX3, pentraxin 3; HD, hospital day. |
Table 1
Comparison of Clinical Characteristics and Sepsis Treatment between Survivors and Non-Survivors

BMI, body mass index; SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation; CRRT, continuous renal replacement therapy; EGDT, early goal-directed therapy; CVP, central venous pressure; MAP, mean arterial pressure; SCVO2, superior vena cava oxygenation saturation.
Values are presented as the median (interquartile range) or number (percent). SOFA score was assessed upon arrival at the emergency department and APACHE II score was assessed based on the worst values over the first 24 hrs of intensive care unit care.
*Except essential hypertension, †Multiple selection was possible in one patient.
Table 2
Comparison of Inflammatory Markers Measured on Hospital day 0, 3, and 7 between Survivors and Non-Survivors
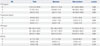
PTX3, pentraxin 3; CRP, C-reactive protein; DNI, delta neutrophil index; HD, hospital day.
Values are presented as the median (interquartile range). The number of total participants who received the measurement of above four inflammatory markers were 83, 78, and 76 at HD 0, 3, and 7, respectively. In survivors, the inflammatory markers were measured in 67 patients at all HD 0, 3, and 7. In non-survivors, the number of patient who received the measurement of the inflammatory markers were 16, 11, and 9 at HD 0, 3, and 7, respectively.
Table 3
The Correlation of PTX3 with Other Inflammatory Markers Measured in All Participants Who were Alive at Each HD

PTX3, pentraxin 3; CRP, C-reactive protein; DNI, delta neutrophil index; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; HD, hospital day.
r refers to the Pearson's correlation coefficient. SOFA score was assessed upon arrival at the emergency department and APACHE II score was assessed based on the worst values over the first 24 hrs of intensive care unit care. The number of patients at HD 0, 3, 7, and 14 were 83, 78, 76, and 70, respectively.
Table 4
Independent Predictive Factors for 28-Day All-Cause Mortality in Severe Sepsis Patients Receiving Successful Early-Goal Directed Therapy





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download