Abstract
Purpose
Circumferential pulmonary (PV) vein isolation (CPVI) is the most important treatment strategy for atrial fibrillation (AF). While understanding left atrial wall thickness around PVs (PVWT) prior to catheter ablation is important, its clinical implications are not known. This study aimed to evaluate PVWT characteristics according to underlying disease and to identify associations between PVWT and reconnections of PV potentials (PVPs) in redo ablation.
Materials and Methods
In 28 patients who underwent redo-AF ablation, PVWT and reconnected PVPs were evaluated at 12 sites (1–12 o'clock) around each PV. Clinical characteristics including stroke and CHA2DS2-VASc scores were analyzed according to the PVWT.
Results
The PVWT was thicker in males than females (p<0.001) and in those with diabetes (p=0.045) or heart failure (p=0.002) than in those without. Patients with strokes or high CHA2DS2-VASc scores (≥3) had significantly thinner PVWTs than those without strokes or low CHA2DS2-VASc scores (p<0.001). In redo-ablation, reconnected PVPs were detected in 60 (53.6%) of 112 PVs, and the PVs were thicker (p<0.001) and had more reconnected PVs (p=0.009) than right PVs. A PVWT of >0.6 mm predicted PV reconnections with a sensitivity of 76.7% and specificity of 52.2% with an area under the curve of 0.695.
Treatment of atrial fibrillation (AF) has made continuous advancements since the isolation of ectopic foci in pulmonary veins (PVs) was introduced.1 Eliminating AF triggers in PVs has become a fundamental procedure in patients with AF. However, as AF lasts longer, atrial remodeling progresses, and non-PV atrial substrate plays an important role in arrhythmic foci. Thus, a strategy to modify left atrial (LA) substrate was introduced by Nademanee who demonstrated the clinical implications of catheter ablation of complex fractionated atrial electrograms (CFAEs).2 Further, it has been reported that catheter ablation of CFAEs with PV isolation (PVI) reduces clinical recurrence and repeat procedures in patients with persistent AF (PeAF).3 We also previously reported an association between CFAEs and LA wall thickness (LAWT).45 Meanwhile, however, the mechanism of CFAEs is still controversial and an additional line ablation for reducing the critical mass of LA substrate has not shown any additive effects.6 As a consequence, PVI still plays the most important role in preventing AF progression and reconnection of PV potentials (PVPs).
A perfect PVI is affected by anatomical variations in the PVs, exact catheter contact, and whether or not there is an adequate energy delivery to the endocardium. The development of 3-dimensional cardiac computed tomography (3D-cCT) images made it possible to confirm the LA structure, anatomy, and thickness beforehand. In our previous study, we showed an association between change in LA substrate and the presence of CFAEs.45 Therefore, “tailor-made ablation” according to wall thickness may be possible by confirming LA anatomical variation and antral wall thickness around PVs (PVWT) beforehand, potentially improving catheter ablation outcomes. The purpose of this study was to evaluate characteristics of PVWT according to underlying disease and to identify associations between PVWT and reconnections of PVPs in patients undergoing redo-ablation.
The study protocol was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System. All patients provided written informed consent. The study included 28 non-valvular paroxysmal AF (PAF) patients (22 males, mean age 53.6±12.4 years) who underwent a redo-ablation procedure due to recurrent AF after radiofrequency catheter ablation (RFCA) of drug-refractory AF between January 2009 and December 2012. All patients maintained an optimal anticoagulation (target INR 2.0–3.0) before the procedure, and all antiarrhythmic drugs were discontinued for at least five half-lives of each drug and for at least 4 weeks, especially amiodarone. We examined all patients with transthoracic echocardiography (TTE) and 3D-cCT before the first ablation for AF in order to measure the PVWT and to define LA anatomy before the RFCA.
All patients underwent 3D-cCT before RFCA of PAF. All contrast-enhanced 3D-cCT examinations were performed using a second generation dual-source CT scanner (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany). A contrast agent (Iopamiro 370; 370 mg iodine/mL, Bracco, Milan, Italy) was injected using a power injector (Envision CT, Medrad, Warrendale, PA, USA) at a flow rate of 5 mL/s into the right antecubital vein with the triple phase injection method. Following the administration of 60–80 mL of a contrast agent, 30 mL of a 70:30 saline-to-contrast mixture and 20 mL of pure saline were administered at a flow rate of 5 mL/s through the same venous access. Scanning was performed with the following parameters: prospective electrocardiography (ECG)-gated axial acquisition targeting the end-systolic phase using the absolute delay method, detector configuration of 2×64×0.6 mm (acquisition of 2×128 sections per rotation using a z-axis flying focal spot), gantry rotation time of 0.285 s, tube potential of 80–120 kVp, and tube current-time product of 280–450 mAs, depending on the patient's body mass index. The scan delay time was determined by the test-bolus technique for optimal contrast enhancement. After injection of a 10-mL bolus of iopamiro, the optimal delay times were determined by automatic detection of contrast enhancement in the ascending aorta. Reconstructed images were transferred to an image server and analyzed using commercially available 3D software (Aquarius iNtuition, Ver 4.4.6, TeraRecon, San Mateo, CA, USA).
A radiologist blinded to the patients' clinical and electrophysiological information evaluated the CT images independently. The intra- and inter-observer correlation thereof was already confirmed in a previous study.45 For the measurement of the PVWTs, all CT images were transferred to commercially available 3D reconstruction software (Aquarius iNtuition, Ver 4.4.6, TeraRecon), and then multiplanar (MPR) images were reconstructed. Using axial and coronal images, oblique axial images and oblique coronal images were obtained, which were parallel to the target PV passage. Then, a true enface view of the target PV was obtained, which was simultaneously perpendicular to the previous oblique axial and oblique coronal images. The lung parenchyma and fat tissue were automatically masked from the PVs using an auto-segmentation technique with a default range from -1024 to -45 Hounsfield unit (HU). On the enface view, the PVWTs were measured carefully at 12 equiangular points (1 o'clock to 12 o'clock sites) using electrical calipers. The PVWT was measured with the Aquarius iNtuition software. First, the oblique axial images (Fig. 1A) and oblique coronal images (Fig. 1B) were obtained, which were parallel to the target PV passage (right superior PV) using axial and coronal images (not shown here). Then, a true enface view of the target PV (Fig. 1C) was obtained, which was simultaneously perpendicular to the previous oblique axial (Fig. 1A) and oblique coronal (Fig. 1B) images. The lung parenchyma and fat tissue were automatically masked from the target PV using an auto-segmentation technique with a default range from -1024 to -45 HU (blue area). On the enface view, the PVWTs were measured carefully at 12 equiangular points (1 o'clock to 12 o'clock sites) using electrical calipers (Fig. 1D). The LA antrum around the PVs (PVW) was defined as the region consisting of a 10-mm space between the PV ostium and left atrium.
All patients underwent circumferential PVI (CPVI) and cavotricuspid isthmus block without an additional line or CFAE ablation in the first ablation for PAF. An open irrigated tip, 3.5-mm-tip deflectable catheter (Thermocool, Johnson & Johnson Inc., Diamond Bar, CA, USA; Coolflex, St. Jude Medical Inc., Minnetonka, MN, USA) was used for the RFCA (Stockert generator, Biosense Webster Inc.; Diamond Bar, CA, USA; Irvine Biomedical, Inc., -1500T11 generator, St. Jude Medical Company, CA, USA). All patients were followed-up in the outpatient clinic without the use of any antiarrhythmic drugs after the RFCA. The patients visited an outpatient clinic regularly at 1, 3, 6, and 12 months and then every 6 months or whenever symptoms occurred after the RFCA. All patients underwent an ECG at every visit and a 24- or 48-hour Holter recording and/or event recording at 3, 6, and every 6 months, following the 2012 HRS/EHRA/ECAS Expert Consensus Statement guidelines. However, whenever patients reported palpitations, Holter monitor or event monitor recordings were obtained and evaluated for possible recurrences of arrhythmias. We defined a recurrence of AF as any episode of AF or atrial tachycardia lasting at least 30 sec.7 Any ECG documentation of AF recurrences after 3 months was diagnosed as a clinical recurrence,7 and we performed a redo-ablation for the patients who had a clinical recurrence of AF. At the time of the redo-ablation procedure, we first evaluated the presence of reconnected PVPs with a variable loop circular mapping catheter (LASSO®, Biosense Webster Inc., Diamond Bar, CA, USA) of each PV. The sites of reconnected potentials were marked in a clockwise rotation from 1 to 12 (Figs. 1D and 5), and we generated 3D-cCT VR images with an electroanatomical mapping system (Ensite NavX system, St. Jude Medical Inc., Minneapolis, MN, USA) for exact matching with the 3D-cCT and PVP site.
All patients underwent TTE (Sonos 5500, Philips Medical System, Andover, MA, USA or Vivid 7, GE Vingmed Ultrasound, Horten, Norway) prior to the RFCA. The chamber size (LA volume index, LA dimension, LV wall thickness, and LV mass index), transmitral flow velocity (E wave, A wave), and tissue Doppler images of the mitral annular septal area (peak diastolic velocity and peak systolic velocity) were acquired according to the American Society of Echocardiography guidelines.89 The index was calculated as divided by the body surface area.
Normally-distributed continuous variables are expressed as the mean±standard deviation. The statistical significance of comparisons was assessed using Student's t-test, χ2 test, and ANOVA test. A receiver operating characteristic (ROC) curve analysis was conducted to evaluate the prognostic value of PVWT in relation to reconnections of the PVPs. A p-value<0.05 was considered statistically significant.
A total of 28 patients (78.6% male, 53.6±12.4 years) were enrolled. Their mean CHA2DS2-VASc score was 1.4±1.3 with a mean LA dimension of 39.6±6.5 mm. The mean wall thickness of the 112 PVs (4 PVs×28 patients) was 0.64±0.25 mm (range 0.13–2.57 mm). Left PVs were thicker than the right PVs (0.67±0.24 mm vs. 0.61±0.26 mm, p<0.001) and had more reconnected PVs (57.1% vs. 50.0%, p=0.009). However, there was no significant difference between the superior and inferior PVs (Table 1).
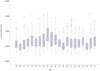
Generally, the PVW was thickest in those in their mid-fifties; however, there was no significant difference in the PVWT in the age group analysis according to a cut-off value of 65 years (p>0.05) (Fig. 2). Male patients had a greater increased PVWT than female patients (0.65±0.26 mm vs. 0.58±0.20 mm, p<0.001) (Fig. 3A). Patients with heart failure (0.70±0.23 mm vs. 0.63±0.25 mm, p=0.002) and diabetes (0.67±0.28 mm vs. 0.63±0.24 mm, p=0.045) had an increased PVWT, compared to those without (Fig. 3B, C, and D). Interestingly, the patients with stroke had a significantly decreased PVWT (0.53±0.18 mm vs. 0.65±0.25 mm, p<0.001), and patients with a high CHA2DS2-VASc score (≥3) also had a decreased PVWT (0.59±0.26 mm vs. 0.65±0.25 mm, p=0.005) (Fig. 4).
In the redo-ablation procedures, reconnected PVPs were detected in 60 (53.6%) of the 112 PVs, and the sites with reconnected PVPs were significantly thicker than those without (0.83±0.29 mm vs. 0.63±0.25 mm, p<0.001) (Fig. 5). The area under curve of the ROC curve for predicting PV reconnections was 0.695; moreover, a PVWT of ≥0.6 mm could predict PV reconnections with a sensitivity of 76.7% and specificity of 52.2% (Fig. 6).
In the present study, males exhibited an increased PVWT, compared to females, and PVW was thickest in those in their mid-fifties and became thinner with increasing age. Patients with heart failure or diabetes had an increased PVWT, compared to those without. Meanwhile, patients with stroke or a high CHA2DS2-VASc score (≥3) had a lower PVWT than the other patients. PVWT was associated with advanced LA remodeling related to underlying disease in patients with PAF and may have resulted in the occurrence of a stroke.
Many studies have aimed to predict the outcomes of AF related to LA volume and dimensions.101112 Measurement of the volume has become more exact with the development of echocardiography and 3D-cCT imaging, and even a segmental approach for determining the LA volume has recently become possible. 1314 However, many studies on LA volume by 2D echocardiography have shown consistent underestimation, compared to 3D-cCT, due to ambiguous endocardial borders and geometric assumption in the apical view. Because 3D-cCT is tomographic, the chamber orientation and variations of the cardiac size and dimension during the cardiac cycle will not affect the volume assessments.15 This also allows for fewer geometric assumptions and has the ability to prescribe any plane across the LA. LA remodeling in patients with AF progression usually occurs more in the anteroposterior dimension than LA “elongation”. 13 Remodeling of LA dimensions in AF patients might lead to underestimation of LA volume. Although this limitation is inherent to echocardiography, studies have shown that echocardiography tends to underestimate LA volume and is strongly correlated with 3D-cCT measurements.16 These results were similar to our findings. In addition, because the LA wall is very thin, its measurement by echocardiography is impossible and the development of the 3D-cCT image resolution was necessary for an exact measurement. We already measured the LAWT with 31 segmented sites of the LA and showed an association between LAWT and CFAEs.45 Further, measurements of the LAWT by two independent observers showed an excellent agreement (inraclass correlation coefficient=0.984, p<0.001).45 For the measurement of PVWT in this study, the observers determined the points for measurement around the PVs using the three-dimensional volume-rendered images and MPR reformatted images. If the inner and outer borders of the target wall were determined, the PVWT was measured using electronic calipers with the same method as the previous study.
Since CPVI has been an important milestone of the treatment of AF,1 its clinical importance has increased even in patients with PeAF.6 A complete CPVI and its maintenance is considered to play an important role in the prevention of AF recurrence regardless of AF type. Thus, it is very important to make a transmural lesion and complete block around all four PVs after evaluating the anatomical variation of each PV. However, most previous studies have focused on geometric variation and exact contact, and there has been no interest regarding the proper amount of energy delivery according to wall thickness: high energy delivery may be needed for a thick wall, in terms of a transmural lesion formation, and a relatively low energy delivery could be adequate for a thin wall, considering the risk of perforation. Generally, the disappearance of PVPs and an increased impedance are considered as endpoints of the ablation at each site.17 With this strategy, the information on the wall thickness enables a tailored ablation and energy delivery at each site. In this study, the reconnection of PVPs was noted more frequently at sites with a thick wall after the same ablation strategy, and this finding indicated that tailored ablation according to wall thickness may be necessary. Moreover, with continuous information, not point-by-point information, on the PVWT can be acquired, an exact tailored ablation can become possible and could reduce PV reconnections and the risk of ablation.
In this study, males had a thicker wall around PVs than females, although there was no significant difference according to age therein. Further, patients with heart failure or diabetes had a thicker wall around the PVs than those without. Heart failure with a decreased LV function results in a volume overload and pressure increase in the LA, and this change may cause an increase in the PVWT and LA wall hypertrophy. Further, pathological changes in the myocardium of patients with diabetes have already been reported, even though it was without ventricular systolic decompensation. The reported changes included myocardia fibrosis,18 deposition of periodic acid-Schiff-positive material, advanced glycation endproduct deposition,19 and capillary basement membrane changes.20 As a consequence, those pathological alterations resulted in an increase in the myocardial stiffness, LV mass, and wall thickness.21 Interestingly, we found that patients with stroke or a high CHA2DS2-VASc score had a thin wall around the PVs. In previous studies, patients with PAF exhibited a thicker LA wall than those with normal sinus rhythm, although as AF progressed to chronic AF, the LA wall became thin.22 The PVWT reflects LA remodeling from AF progression, and as a consequence, a thin PVWT may be associated with the occurrence of a stroke due to a high CHA2DS2-VASc score, as well as a previous stroke. In the beginning of AF, risk factors, such as heart failure and diabetes, may increase the wall thickness around the PVs; however, the PVWT will become decreased as the LA remodeling progresses. As a result, a thin PVWT may be associated with the occurrence of a stroke. Therefore, if measurement of LA PVWT is possible, a more exact prediction of a stroke due to AF could potentially be accomplished.
This study had some limitations. First, we focused on reconnections of the CPVI in patients with PAF and could not analyze the reconnections and bidirectional block of the linear ablation in those with PeAF, which are related to wall thickness. Second, this study had a small number of enrolled patients. Third, the reconnection sites of the PVPs during the procedure and the PVWT obtained by 3D-cCT could not be simultaneously accomplished. Further, measurements around the left PVs with the ridge anteriorly are difficult. To overcome this limitation, we generated cardiac 3D-cCT VR images with an electroanatomical mapping system for an exact matching of the 3D-cCT and PVP site. Fourth, the PVWTs were measured at just 12 points around each PV, and continuous measurements should be performed in a future study. Finally, we used a low ablation power when ablating the posterior wall to avoid any esophageal injury, and could not show any significant correlation between the PVWT of the anterior and posterior regions according to the reconnection sites.
In conclusion, patients with stroke or a high CHA2DS2-VASc score had a thinner PVW than those without, and the PVWT may be a predictor of a stroke occurrence, as well as previous stroke related to LA remodeling due to AF. Further, a thick PVW was associated with male patients, diabetes, heart failure, and reconnection of PVPs after CPVI.
Figures and Tables
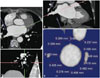 | Fig. 1Measurement of the PVWT (left atrial antral wall thickness around the right superior pulmonary vein). The details are described in the Methods section. (A) oblique axial images, (B) oblique coronary images, (C) true enface view of the target pulmonary vein, (D) the PVWTs are measured carefully at 12 equiangular points (1 o'clock to 12 o'clock sites). PVWT, wall thickness around pulmonary veins. |
 | Fig. 2The distribution of the PVWT according to age. The PVW was thickest in those in their mid-fifties and became thinner with increasing age. PVWT, wall thickness around pulmonary veins. |
 | Fig. 3The difference in the PVWT according to the gender (A) and underlying disease, including heart failure (B), hypertension (C), and diabetes (D). *p<0.05. PVWT, wall thickness around pulmonary veins. |
 | Fig. 4The PVWT of the patients with a stroke (A) or high CHA2DS2-VASc score (B) was thinner than that in those without. *p<0.05. PVWT, wall thickness around pulmonary veins. |
 | Fig. 5The PVWT and reconnection of the PV potentials (schematic enface view of each PV from 1 to 12 o'clock). The outer circle (blue) indicates the relative PVWT, and inner circle (orange) the relative ratio of the PV reconnections. The numbers in the parentheses denote the number of PV potentials that were detected at each site. RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; PVWT, wall thickness around PVs; PV, pulmonary vein. |
 | Fig. 6ROC curve of the PWVT for predicting PV reconnections. ROC, receiver operating characteristic; PVWT, wall thickness around PVs; PV, pulmonary vein. |
Table 1
Baseline Characteristics of the Enrolled Patients

ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2012R1A2A2A02045367, NRF-2015R1C1A1A02037085), the Ewha Womans University Research Grant (1-2016-0308-001-1) of 2016, and grants from the Korean Healthcare technology R&D project funded by the Ministry of Health & Welfare (HI12C1552, HI16C0058, HI15C1200).
References
1. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339:659–666.

2. Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004; 43:2044–2053.

3. Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, et al. Substrate and trigger ablation for reduction of atrial fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J. 2010; 31:1344–1356.

4. Park J, Park CH, Lee HJ, Wi J, Uhm JS, Pak HN, et al. Left atrial wall thickness rather than epicardial fat thickness is related to complex fractionated atrial electrogram. Int J Cardiol. 2014; 172:e411–e413.

5. Wi J, Lee HJ, Uhm JS, Kim JY, Pak HN, Lee M, et al. Complex fractionated atrial electrograms related to left atrial wall thickness. J Cardiovasc Electrophysiol. 2014; 25:1141–1149.

6. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015; 372:1812–1822.

7. European Heart Rhythm Association (EHRA). European Cardiac Arrhythmia Scoiety (ECAS). American College of Cardiology (ACC). American Heart Association (AHA). Society of Thoracic Surgeons (STS). Calkins H, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007; 4:816–861.

8. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009; 22:107–133.

9. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463.

10. Parkash R, Green MS, Kerr CR, Connolly SJ, Klein GJ, Sheldon R, et al. The association of left atrial size and occurrence of atrial fibrillation: a prospective cohort study from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2004; 148:649–654.

11. Reant P, Lafitte S, Jaïs P, Serri K, Weerasooriya R, Hocini M, et al. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation. 2005; 112:2896–2903.

12. Beukema WP, Elvan A, Sie HT, Misier AR, Wellens HJ. Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation. 2005; 112:2089–2095.

13. Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, et al. The relationship between endocardial voltage and regional volume in electroanatomical remodeled left atria in patients with atrial fibrillation: comparison of three-dimensional computed tomographic images and voltage mapping. J Cardiovasc Electrophysiol. 2009; 20:1349–1356.

14. Park MJ, Jung JI, Oh YS, Youn HJ. Assessment of the structural remodeling of the left atrium by 64-multislice cardiac CT: comparative studies in controls and patients with atrial fibrillation. Int J Cardiol. 2012; 159:181–186.

15. Avelar E, Durst R, Rosito GA, Thangaroopan M, Kumar S, Tournoux F, et al. Comparison of the accuracy of multidetector computed tomography versus two-dimensional echocardiography to measure left atrial volume. Am J Cardiol. 2010; 106:104–109.

16. Heo R, Hong GR, Kim YJ, Mancina J, Cho IJ, Shim CY, et al. Automated quantification of left atrial size using three-beat averaging real-time three dimensional Echocardiography in patients with atrial fibrillation. Cardiovasc Ultrasound. 2015; 13:38.

17. Haines D. Biophysics of ablation: application to technology. J Cardiovasc Electrophysiol. 2004; 15:10 Suppl. S2–S11.

18. van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990; 82:848–855.

19. van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008; 117:43–51.
20. Fischer VW, Barner HB, Leskiw ML. Capillary basal laminar thichness in diabetic human myocardium. Diabetes. 1979; 28:713–719.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download